Question: please answer both quedtions with units do not use other chegg answers plz thnx Part B: Copper has a FCC structure and an energy for

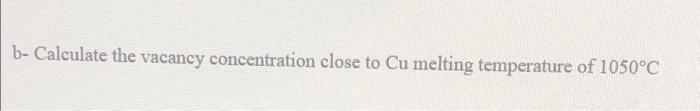
Part B: Copper has a FCC structure and an energy for vacancy formation of 0.9 eV/atom. a- Calculate the number (amount) of vacancies per unit cell for copper at 600C b- Calculate the vacancy concentration close to Cu melting temperature of 1050C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


