Question: PLEASE ANSWER BOTH QUESTIONS 1) a) 6.29 mg/g b) 21.18 mg/g c) 0.135 L/mg d)1.352 L/mg 2) a) M1 b) M2 The figure below depicts
PLEASE ANSWER BOTH QUESTIONS
1)

a) 6.29 mg/g
b) 21.18 mg/g
c) 0.135 L/mg
d)1.352 L/mg
2)

a) M1
b) M2
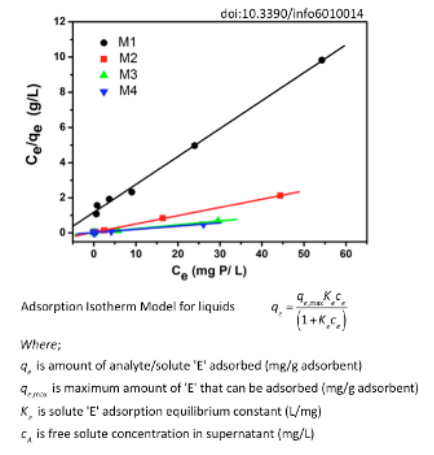
The figure below depicts linearized adsorption isotherms for a run-off fertilizer chemical contaminating ground water (solute E) on four novel adsorbent materials that were designed for solute sequestration/removal by your company. What is the maximum amount of solute E that can be adsorbed per gram of adsorbent material M1 based on the data given below (pick the closest answer from the four options given below)? 12 10 (7/6) bjg M1 M2 AM3 M4 10 20 30 Ce (mg P/L) Adsorption Isotherm Model for liquids (1+k_c.) Where; q, is amount of analyte/solute 'E' adsorbed (mg/g adsorbent) exis maximum amount of 'E' that can be adsorbed (mg/g adsorbent) K, is solute 'E' adsorption equilibrium constant (L/mg) c is free solute concentration in supernatant (mg/L) doi:10.3390/info6010014 40 50 60 8 Continued from problem statement earlier...based on the plots shown below, which of the two adsorbent materials (M1 versus M2) would have a higher qe,max value? Hint: You need to use the linearized Langmuir adsorption model equation to solve this problem! See figure for details. Article Reference: doi:10.3390/info6010014
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


