Question: Please answer both questions (MIN WORDS 400-450 for each question) (example question 1 is 400-450 words and question 2 400-450 words as well) for a







Please answer both questions (MIN WORDS 400-450 for each question) (example question 1 is 400-450 words and question 2 400-450 words as well) for a like and good rating!!! THANKS :)
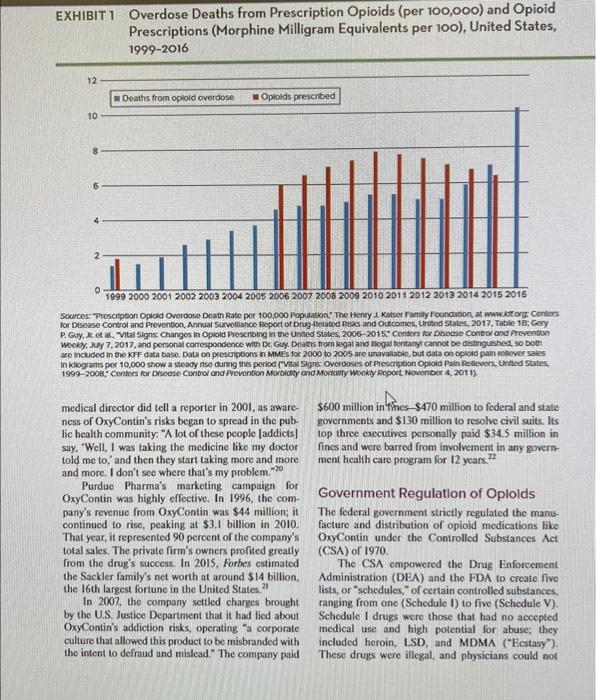
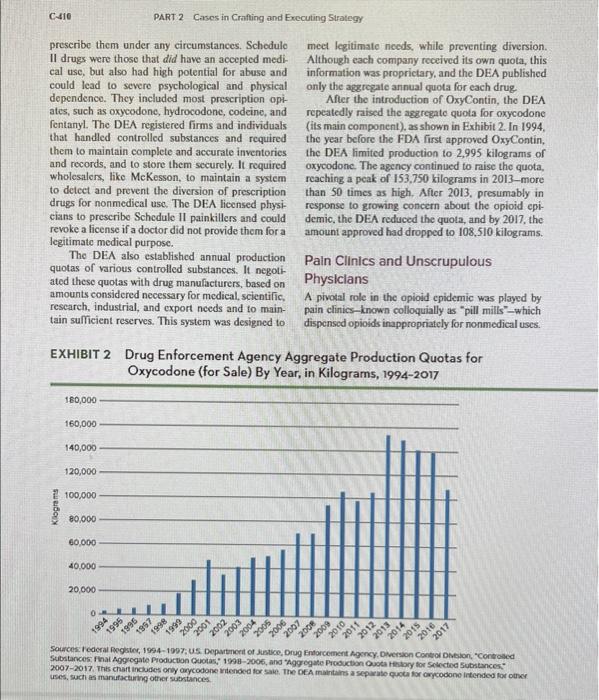
n 2017, McKesson Corporation, a leading wholesale drug distributor, agreed to pay $150 million in fines to the U.S. Department of Justice. The charges were that the company had failed to implement effective controls to prevent the diversion of prescription opioids for nonlegitimate uses, in violation of the Controlled Substances Act.' For example, McKesson had supplied pharmacies in Mingo County, West Virginia-a poor, rural county with the fourth-highest death rate from opioid overdoses in the nation-with 3.3 million more hydrocodone pills in one year than it had in five consecutive earlier years. 2 At the time, Mingo County had just 25,000 residents. Yet, the company had not flagged these orders to federal drug enforcement officials as out-ofthe-ordinary. McKesson, which at the time was the fifth larg. est company in the United States-with almost \$200 billion in annual revenue-played a largely unnoticed middleman role in the pharmaceutical industry. The firm's main business was shipping legal, governmentapproved medicines to pharmacies, hospitals, and health systems. McKesson's unmarked (rucks rolled out at midnight from its 28 enormous, highly automated distribution centers, on route to their morning deliveries of one-third of all pharmaceuticals sold in North America. Although distributors like McKesson did not either manufacture or dispense opioids, they were responsible for notifying the federal Drug Enforcement Administration (DEA) and corresponding state regulators if orders suggested that controlled substances were being improperly diverted. 3 McKesson and other drus distributors were not the only businesses implicated in the nation's burgeoning epidemic of addictive opioids. Drug companies-such as Purdue Pharma, the maker of OxyContin-had developed new prescription opioids and aggressively marketed them to doctors and patients, making vast prolits for their owners. Entrepreneurs had opened pain clinies where unscrupulous doctors could write big scripts for the addictive pills, and pharmacies had looked the other way while dispensing drugs to suspicious patients. And illegal businesses, from producers of streel drugs like heroin to networks of dealers, had also played their parts. What responsibility did these businesses bear for the tragedy of opioid addiction, disability, and death? THE OPIOID EPIDEMIC At the time of the McKesson's settlement with the Justice Department, the United States was deep in the throes of what the Centers for Disease Control and Prevention (CDC) had called "the worst drug overdose epidemic in |U.S.| history. 1 Fucling the epidemic was addiction to preseription opioids. Opioids were a class of painkillers derived from the opium poppy. Also referred to as narcotics, opioids included legal prescription medications such as morphine, codeine, hydrocodone, oxycodone, and fentanyl, as well as illegal drugs such as heroin. Opioids worked by dulling the sensation of pain. At high doses, they could also cause feelings of intense euphoria. The journalist John Temple, author of the investigative report American Pain, described EXHIBIT 1 Overdose Deaths from Prescription Opioids (per 100,o0) and Opioid Prescriptions (Morphine Milligram Equivalents per 100), United States, 19992016 Sources: "Prescilpson Opidid Overdose Death Rate per 100,000 Population:" The Henry 1 Katses Family foundabon, at wwwittorg. Centers P. Guy, Je et al, "Vital signs: Changes in Opiold proscribing in the Unied States, 2006-2015, Contess for Detiease Control ond Preventibn Whekiy, Juy 7,2017, and personal cortespondence with De Guy. Deatrs fromilegal and itogas fentany cannot be disthgulshed, so both are included in the kfF data base. Data on prescipbons in MMF's for 2000 to 2005 are unavathbie, but data co oplove pain setever saies 1999 -2008, Centevs for Degease Control and Freveribon Mortidty ond Movtally Whokly Report Novenber 4, 201 1). medical director did tell a reporier in 2001, as awareness of OxyContin's risks began to spread in the public health community: "A lot of these people [addicts] say. 'Well, I was taking the medicine like my doctor told me to, and then they start faking more and more and more, I don't see where that's my problem. 70 Purdue Pharma's marketing campaign for OxyContin was highly effective. In 1996, the company's revenue from OxyContin was $44 million; it continued to rise, peaking at $3,1 billion in 2010 . That year, it represented 90 percent of the company's total sales. The private firm's owners profited greatly from the drug's success. In 2015, Forbes estimated the Sackler family's net worth at around $14 billion. the I6th largest fortane in the United States. 21 In 2007, the company settled charges brought by the U.S. Justice Depariment that it had tied about OxyContin's addiction risks, operating "a corporate culture that allowed this product to be misbranded with the intent to defraud and mislead." The company paid $600 miltion in hines $470 million to federal and state governments and $130 million to resolve civil suits. Its top three executives personally paid $34.5 million in fines and wero barred from involvement in any government health care program for 12 years. 22 Government Regulation of Oploids The federal government strictly regulated the manufacture and distribution of opioid medications like OxyContin under the Controlled Substances Act (CSA) of 1970. The CSA empowered the Drug Enforcement Administration (DEA) and the FDA to create five lists, or "schedules," of certain controlled substances, ranging from one (Schedule I) to five. (Schedule V). Schedule I drugs were those that had no accepled medical use and bigh potential for abuse; they included heroin, LSD, and MDMA ("Eestasy"). These drugs were illegal, and physicians could not prescribe them under any circumstances. Schedulc II drugs were those that did have an accepted medical use, but also had high potential for abuse and could lead to severe psychological and physical dependence. They included most preseription optates, such as oxycodone, hydrocodone, codcine, and fentanyl. The DEA registered firms and individuals that handled controlled substances and required them to maintain complete and accurate inventories and records, and to store them securely. It required wholesalers, like MeKesson, to maintain a system to deteet and prevent the diversion of prescription drugs for nonmedical use. The DEA licensed physicians to prescribe Schedule II painkillers and could revoke a ficense if a doctor did not provide them for a legilimate medical purpose. The DEA also established annual production quotas of various controlled substances. It negotiated these quotas with drug manufacturers, based on amounts considcred necessary for medical, scientific, rescarch, industrial, and cxport nceds and to maintain sufficient reserves. This system was designed to meet legitimate needs, while preventing diversion. Although each company received its own quota, this information was proprictary, and the DEA published only the agzrezale annual quota for each drug. Nfter the introduction of Oxy Contin, the DEA repeatedly raised the agzregate quota for oxycodone (its main component), as shown in Exhibit 2. In 1994, the year before the FDA first approved Oy Contin, the DEA limited production to 2,995 kilograms of axycodone. The agency continued to raise the quota, reaching a peak of 153,750 kilograms in 2013-more than 50 times as high, After 2013. presumably in response to growing concern about the opioid epidemic, the DEA reduced the quota, and by 2017 , the amount approved had dropped to 108,510 kilograms. Pain Clinics and Unscrupulous Physiclans A pivotal role in the opioid cpidemic was played by pain clinics-kncwn colloquially as "pill mills"-which dispensed opioids inappropriately for nonmedical uses. EXHIBIT 2 Drug Enforcement Agency Aggregate Production Quotas for uses, such it manufacturing otrier signtances. and Walgreens-and callod for them to reimburse the Cherokees for health care costs. "The resources of the Cherokee Nation are being spent on this crisis that otherwise should be spent on our ordinary, cveryday health care needs," suid the Cherokee Nation's attorney general. 16 Some legal experts thought these lawsuits had little chance of success. Prescription opioids had a legitimate medical purpose and had been approved by the government. One expert in product liability law put it this way: "[The distributors] ship a drug that's approved by the FDA, and then a bunch of bad actors intervene-pill mills, doctors who overprescribe, and the addicts themselves. It's a pretty strong argument." Other legal experts, however. thought the companies were in a weaker position. "[The pharmaceutical firms] are big companies that knew their product was doing harm," said an attorney who had been involved in the tobacco liw. suits years eariier. "Instead of helping to solve the problem, they promoted the irresponsible use of their product to improve their bottom line." Added the attorney who represented the Cherokee Nation: "These pharmaceutical companies should bo scared as hell. =37 Assignment Questions 1. How would you describe the pharmaceutical industry's strategy leading up to the opioid crisis? Is it ethical? Why or why not? 2. Is there anything that the pharmaceutical industry has done or is now doing in the aftermath of that crisis that could legitimately be considered as "unethical" by its stakeholders? What grade would you assign to the industry for its handling of the crisis? Explain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


