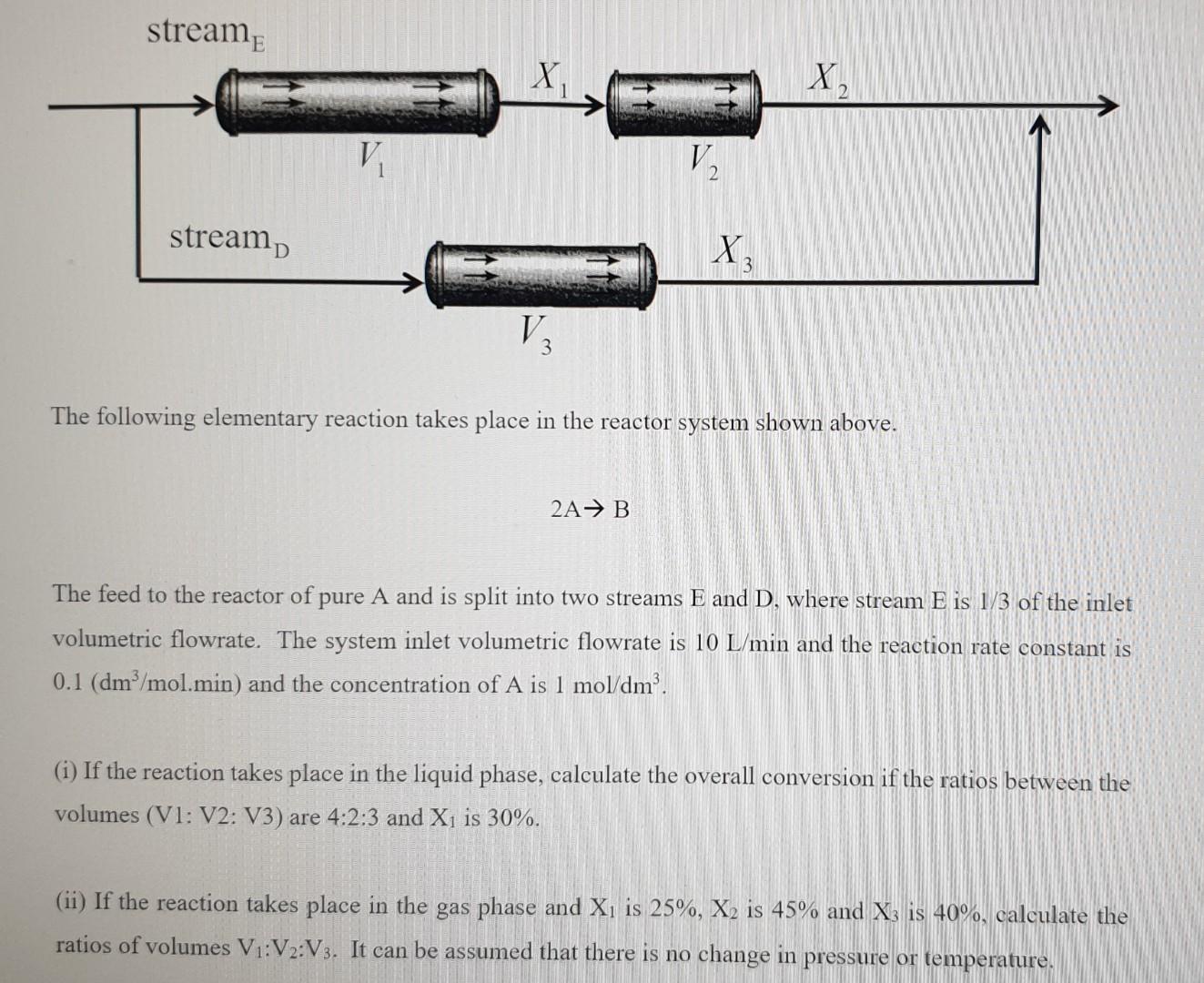
Question: Please answer both questions. stream X x 2 V. V2 stream X 3 V, 3 The following elementary reaction takes place in the reactor system
Please answer both questions.
stream X x 2 V. V2 stream X 3 V, 3 The following elementary reaction takes place in the reactor system shown above. 2A B The feed to the reactor of pure A and is split into two streams E and D, where stream E is 1/3 of the inlet volumetric flowrate. The system inlet volumetric flowrate is 10 L/min and the reaction rate constant is 0.1 (dm /mol.min) and the concentration of A is 1 mol/dm'. (i) If the reaction takes place in the liquid phase, calculate the overall conversion if the ratios between the volumes (V1: V2: V3) are 4:2:3 and Xi is 30%. (ii) If the reaction takes place in the gas phase and Xi is 25%, X, is 45% and X3 is 40%, calculate the ratios of volumes VI:V2:V3. It can be assumed that there is no change in pressure or temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


