Question: please answer both questions Write the net ionic equation for the following molecular equation. Cr(NO3)2(aq)+K2CO3(aq)CrCO3(s)+2KNO3(aq) (Use the lowest possible coefficients, Be sure to specify states
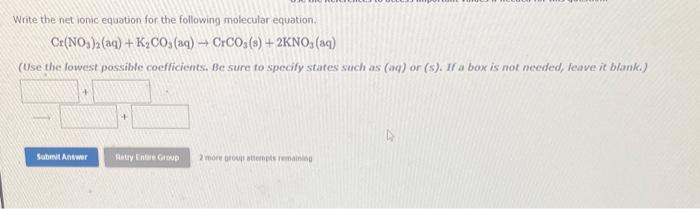

Write the net ionic equation for the following molecular equation. Cr(NO3)2(aq)+K2CO3(aq)CrCO3(s)+2KNO3(aq) (Use the lowest possible coefficients, Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) 2 moore aroup atiemphs remainiog The folkowing molecularequation represents the reaction that occurs when aqueous solutions of sifver(I) nitrate and magnesium chloride are combined. 2AgNO3(aq)+MgCl2(aq)2AgCla)+Mg(NO3)2(aq) Write the batanced net ionic equation for the reaction. (Use the kowest possible coefficients. lle sure to specify states such as (ag) or (s). ff a box is not meeded, fenve it blank.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


