Question: please answer in clear hand writing References Use the References to access important values if needed for this question. The vapor pressure of liquid acetone,
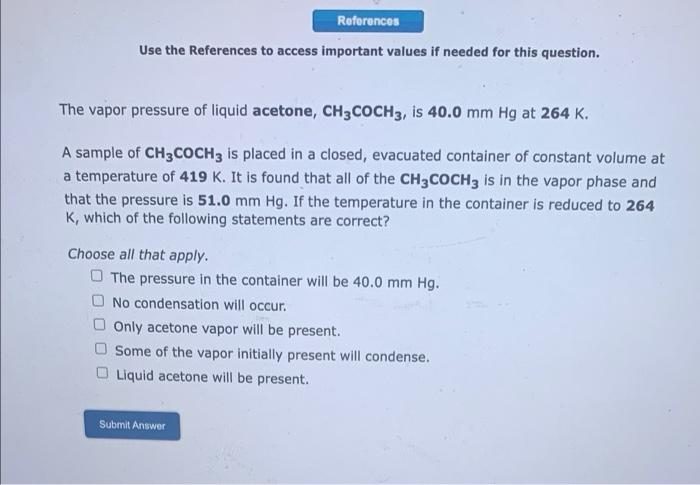
References Use the References to access important values if needed for this question. The vapor pressure of liquid acetone, CH3COCH3, is 40.0 mm Hg at 264 K. A sample of CH3COCHz is placed in a closed, evacuated container of constant volume at a temperature of 419 K. It is found that all of the CH3COCHz is in the vapor phase and that the pressure is 51.0 mm Hg. If the temperature in the container is reduced to 264 K, which of the following statements are correct? Choose all that apply. The pressure in the container will be 40.0 mm Hg. No condensation will occur. Only acetone vapor will be present. Some of the vapor initially present will condense. Liquid acetone will be present. Submit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


