Question: please answer in clear hand writing References Use the References to access important values if needed for this question. The vapor pressure of liquid octane,
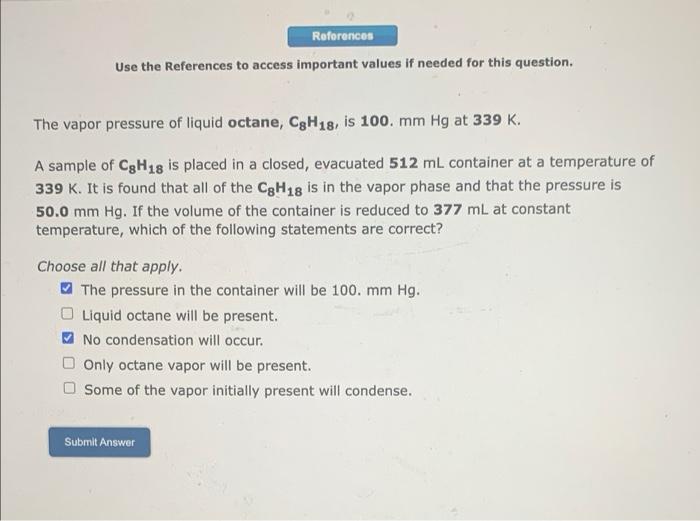
References Use the References to access important values if needed for this question. The vapor pressure of liquid octane, C3H18, is 100. mm Hg at 339 K. A sample of C3H18 is placed in a closed, evacuated 512 mL container at a temperature of 339 K. It is found that all of the C3H18 is in the vapor phase and that the pressure is 50.0 mm Hg. If the volume of the container is reduced to 377 mL at constant temp rature, which of the following statements are correct? Choose all that apply. The pressure in the container will be 100. mm Hg. Liquid octane will be present. No condensation will occur. Only octane vapor will be present. Some of the vapor initially present will condense. Submit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


