Question: PLEASE ANSWER IT CORRECTLY AND TIDILY 2. Component A diffuses through a stagnant film containing only A and B. Upon reaching the catalytic surface, it
PLEASE ANSWER IT CORRECTLY AND TIDILY

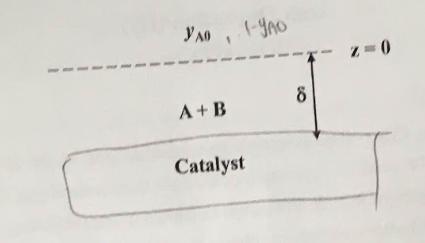
2. Component A diffuses through a stagnant film containing only A and B. Upon reaching the catalytic surface, it is instantaneously converted into species B by the reaction AB. When B diffuses back into the stagnant film, it begins to decompose by the first-order reaction B A. The rate of formation of component A with the film is equal to RA = kus, moles A produced (time)(volume), where yn is the concentration of B expressed in mole fraction. (a) Find the concentration profile of component B in the stagnant film if this is a steady-state process. (10%) (b) Determine the rate at which A enters the gas film (5%) (c) Reconsider the problem and determine the concentration profile of component A in the stagnant film i in the film B decomposes to form A and if A reacts to form B, both by first-order reactions B AF MA: 0 Simultaneously, A is instantaneously reacting to form B in the flat catalytic surface. (10%) 80 1-YAO 8 A+B Catalyst
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


