Question: PLEASE ANSWER IT CORRECTLY AND TIDILY 5. A gas absorption process is used to remove ammonia (NH3) from a gaseous mixture of ammonia and air,
PLEASE ANSWER IT CORRECTLY AND TIDILY
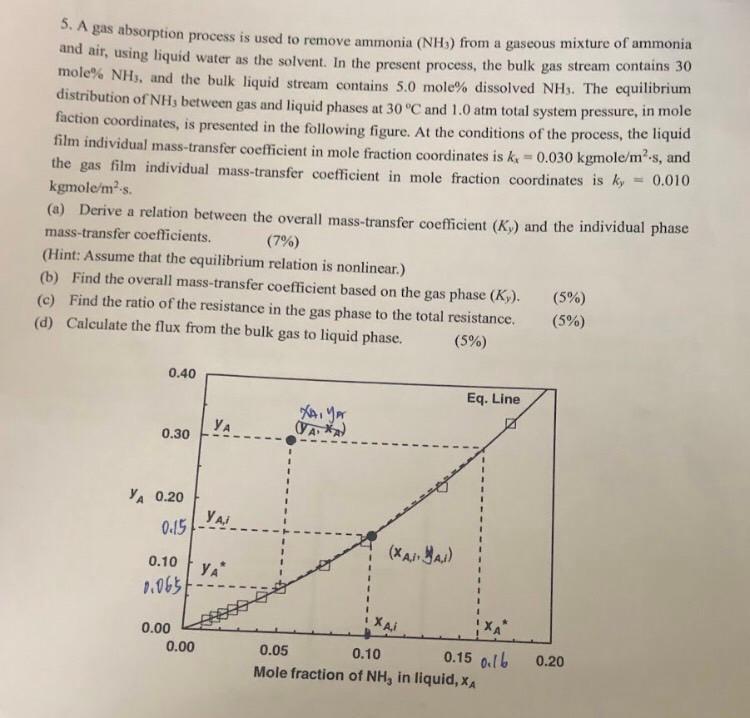
5. A gas absorption process is used to remove ammonia (NH3) from a gaseous mixture of ammonia and air, using liquid water as the solvent. In the present process, the bulk gas stream contains 30 mole% NHs, and the bulk liquid stream contains 5.0 mole% dissolved NHs. The equilibrium distribution of NH, between gas and liquid phases at 30 C and 1.0 atm total system pressure, in mole faction coordinates, is presented in the following figure. At the conditions of the process, the liquid film individual mass-transfer coefficient in mole fraction coordinates is kx -0.030 kgmole/m-s, and the gas film individual mass-transfer coefficient in mole fraction coordinates is ky = 0.010 kgmole/ms. (a) Derive a relation between the overall mass-transfer coefficient (KY) and the individual phase mass-transfer coefficients. (7%) (Hint: Assume that the equilibrium relation is nonlinear.) (b) Find the overall mass-transfer coefficient based on the gas phase (Ky). (5%) (c) Find the ratio of the resistance in the gas phase to the total resistance (5%) (d) Calculate the flux from the bulk gas to liquid phase. (5%) 0.40 Eq. Line yer 0.30 TAX YA 0.20 / 0.15 0.10 YA (XAA.1) 1.065 0.00 0.00 0.05 0.10 0.15 0:16 Mole fraction of NH, in liquid, XA 0.20
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


