Question: Please answer part A and B The conbentrations of reactants and products for a chemical reaction can be caloulated if the equibibum constant tor the
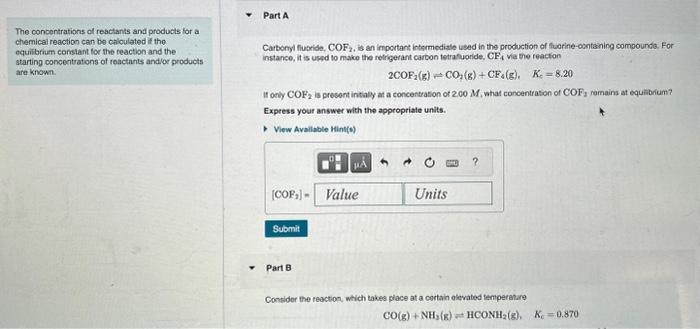
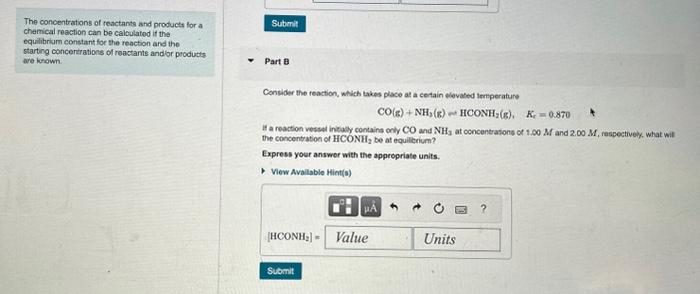
The conbentrations of reactants and products for a chemical reaction can be caloulated if the equibibum constant tor the reaction and the starting concentrations of reactants andlor products Carbonyl fucride, COF2, is an important intarmediale ised in the production of thoerine-osntaning compounde. For instance, it is uspd to make the refrigerant carbon tetrafuoride, CF4 va the reacton are known. 2COF2(g)CO2(g)+CF4(g),Kc=8.20 If only COF2 is presont intialy at a concentration of 2.00M, what concentration of COF2 ramains at equibbrium? Express your answer with the appropriale units. Part 8 Consider the reaction, which takes place at a cortain olevated temperatare CO(g)+NH2(g)HCONH2(g),Kc=0.870 The concentrations of reactants shd products for a chemical reaction can be calculated it the equilibriam constant for the reaction and the starting concentrations of reactamts and or piodutis are krown. Part 8 Consider the teaction, which takes place at a cenain elovated lersperature CO(g)+NH3(g)HCONH2(g),Kr=0.870 If a reaction vestal initaly containa only CO and NH3 at concentrations of 1.00M and 2.00M, respectively. what wil the concentation of HCONH2 be at equillerium? Express your answer with the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


