Question: Please answer the chemical engineering problem accurately and do not copy other's work here or will be downvoted! The rate law for formation of phosgene,
Please answer the chemical engineering problem accurately and do not copy other's work here or will be downvoted!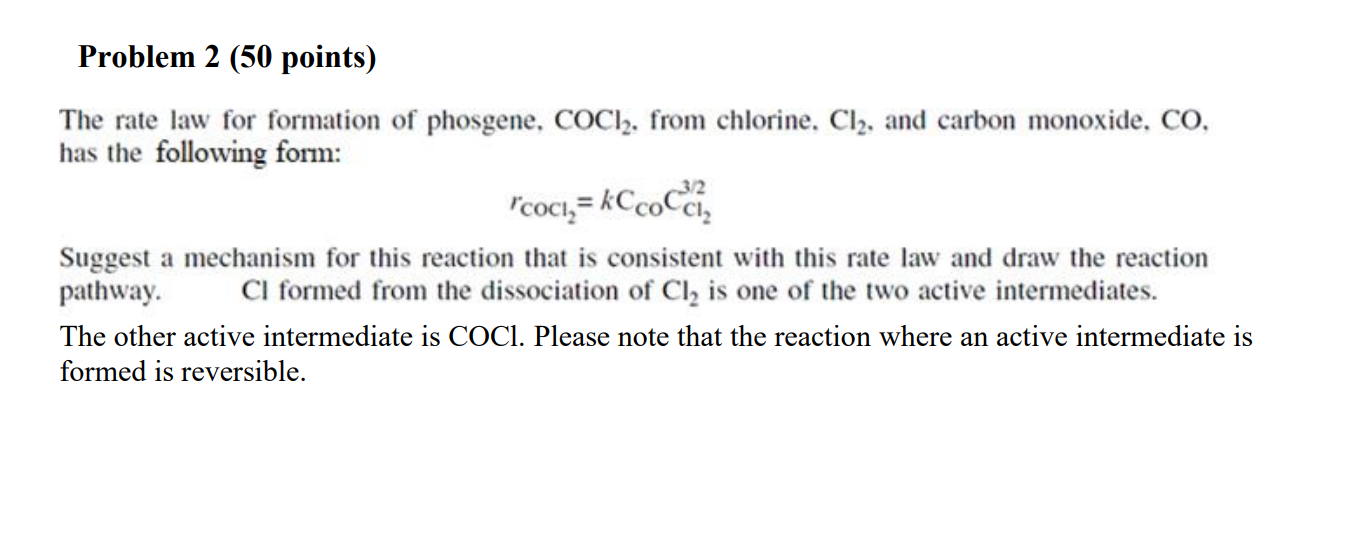
The rate law for formation of phosgene, COCl2, from chlorine, Cl2, and carbon monoxide, CO, has the following form: rCOCl2=kCCOCl23/2 Suggest a mechanism for this reaction that is consistent with this rate law and draw the reaction pathway. Cl formed from the dissociation of Cl2 is one of the two active intermediates. The other active intermediate is COCl. Please note that the reaction where an active intermediate is formed is reversible
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


