Question: Please answer the chemical engineering problem accurately and do not copy other's work here or will be downvoted! Problem 1 (50 points) The following reactions
Please answer the chemical engineering problem accurately and do not copy other's work here or will be downvoted!
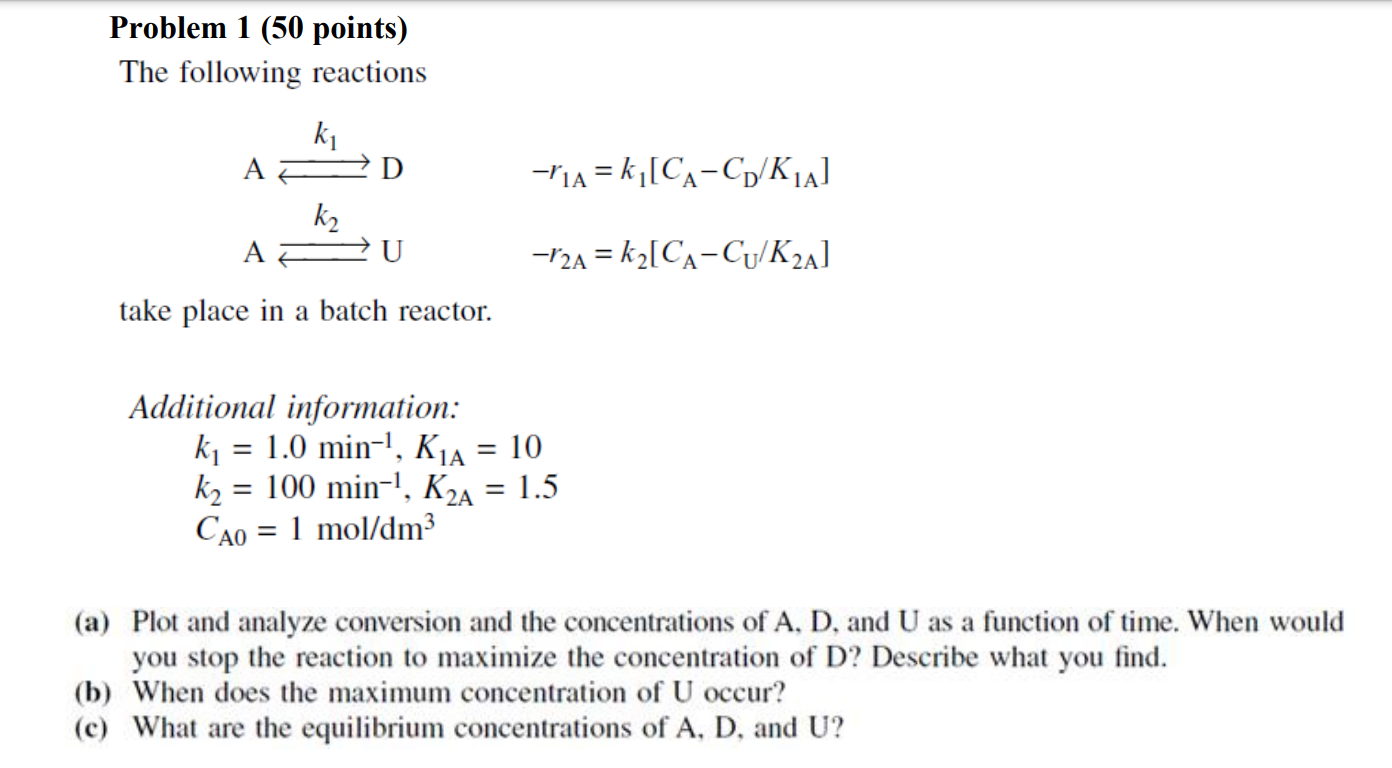
Problem 1 (50 points) The following reactions Ak1LAk2Ur1A=k1[CACD/K1A]r2A=k2[CACU/K2A] take place in a batch reactor. Additional information: k1=1.0min1,K1A=10k2=100min1,K2A=1.5CA0=1mol/dm3 (a) Plot and analyze conversion and the concentrations of A, D, and U as a function of time. When would you stop the reaction to maximize the concentration of D ? Describe what you find. (b) When does the maximum concentration of U occur? (c) What are the equilibrium concentrations of A,D, and U
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


