Question: Please answer the question from the second paragraph. Acetaldehyde is synthesized by the catalytic dehydrogenation of ethanol: C2H5OH CH3CHO + H2 Fresh feed (pure ethanol)
Please answer the question from the second paragraph.
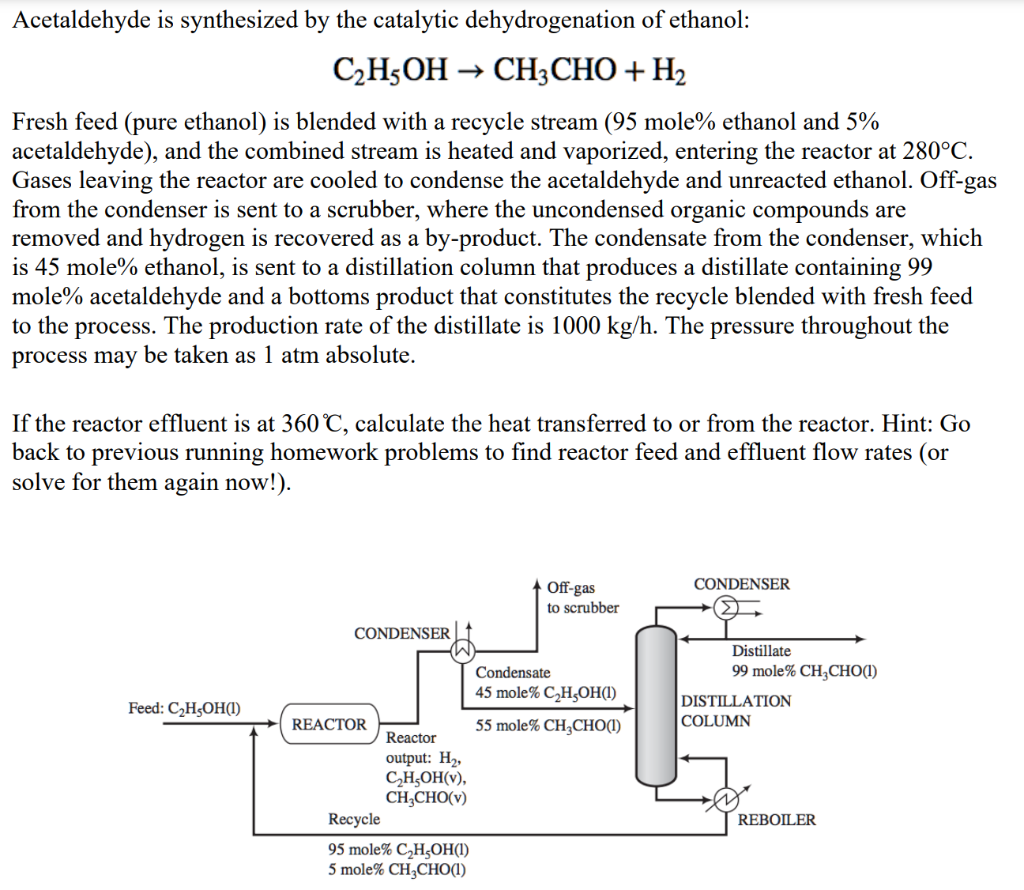
Acetaldehyde is synthesized by the catalytic dehydrogenation of ethanol: C2H5OH CH3CHO + H2 Fresh feed (pure ethanol) is blended with a recycle stream (95 mole% ethanol and 5% acetaldehyde), and the combined stream is heated and vaporized, entering the reactor at 280C. Gases leaving the reactor are cooled to condense the acetaldehyde and unreacted ethanol. Off-gas from the condenser is sent to a scrubber, where the uncondensed organic compounds are removed and hydrogen is recovered as a by-product. The condensate from the condenser, which is 45 mole% ethanol, is sent to a distillation column that produces a distillate containing 99 mole% acetaldehyde and a bottoms product that constitutes the recycle blended with fresh feed to the process. The production rate of the distillate is 1000 kg/h. The pressure throughout the process may be taken as 1 atm absolute. If the reactor effluent is at 360C, calculate the heat transferred to or from the reactor. Hint: Go back to previous running homework problems to find reactor feed and effluent flow rates (or solve for them again now!). Off-gas CONDENSER to scrubber CONDENSER Distillate 99 mole% CHCHO) DISTILLATION COLUMN Feed: C2H5OH(1) Condensate 45 mole% C H2OH(1) REACTOR 55 mole% CHCHO) Reactor output: H2 CH,OH(v), CH,CHO(v) Recycle 95 mole% C,H,OH(1) 5 mole% CHCHO) REBOILER
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


