Question: please answer the two pages. An oxygen gas sample has a volume of 31.0L at 17.0C. What is the volume of this gas sample if
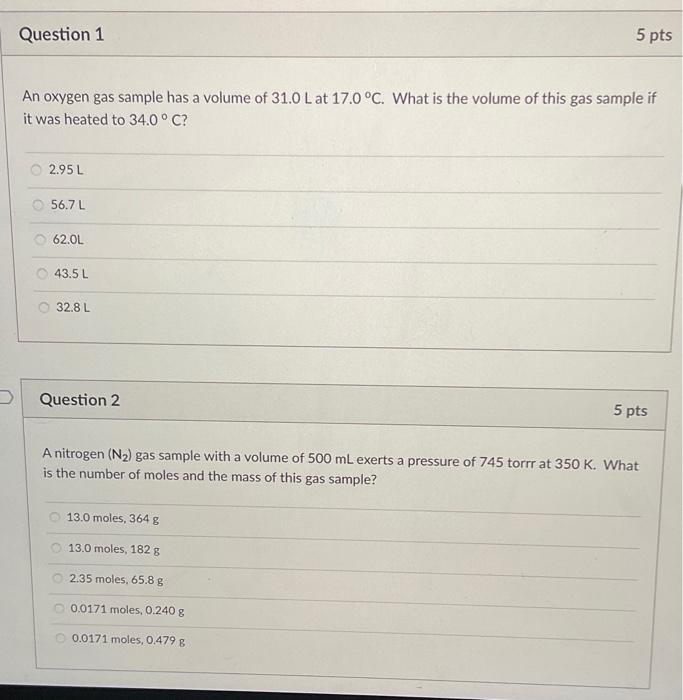
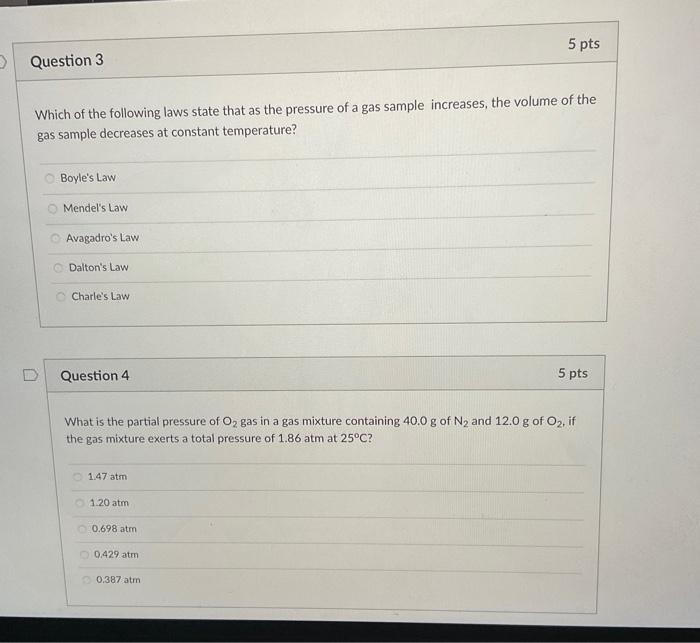
An oxygen gas sample has a volume of 31.0L at 17.0C. What is the volume of this gas sample if it was heated to 34.0C ? 2.95L56.7L62.0L43.5L32.8L Question 2 A nitrogen (N2) gas sample with a volume of 500mL exerts a pressure of 745 torrr at 350K. What is the number of moles and the mass of this gas sample? 13.0 moles, 364g 13.0 moles, 182g 2.35 moles, 65.8g 0.0171 moles, 0.240g 0.0171 moles, 0.479g Which of the following laws state that as the pressure of a gas sample increases, the volume of the gas sample decreases at constant temperature? Boyle's Law Mendel's Law Avagadro's Law Dalton's Law Charle's Law Question 4 5pts What is the partial pressure of O2 gas in a gas mixture containing 40.0g of N2 and 12.0g2O2, if the gas mixture exerts a total pressure of 1.86atm at 25C ? 1.47atm1.20atm0.698atm0.429atm0.387atm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


