Question: please answer them correctly and show all work For a particle in a one-dimensional box, E=n2h2/(8ma2) 1. Use the mass of the electron for m

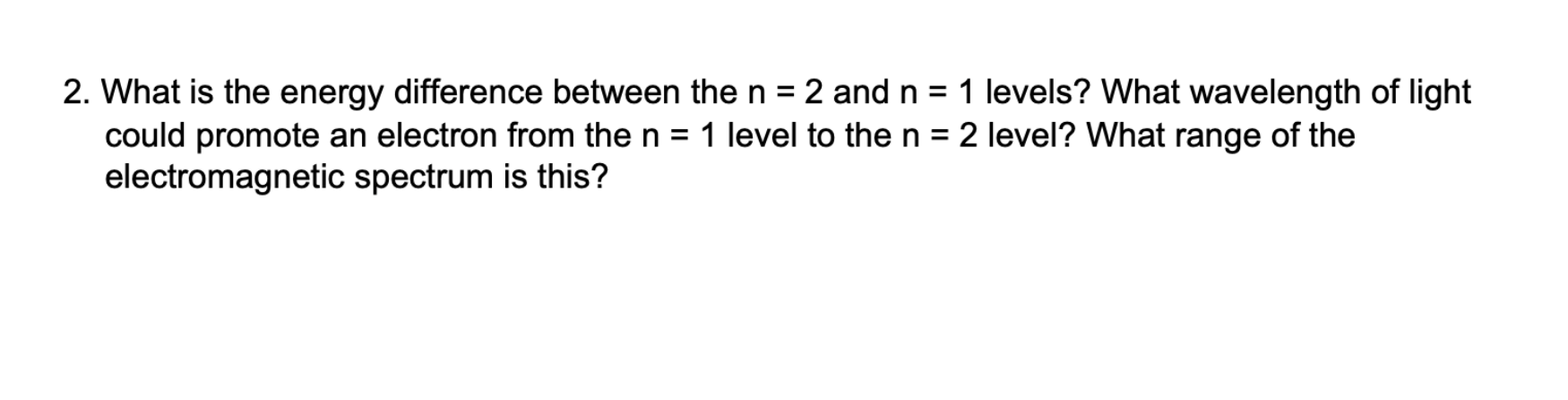 please answer them correctly and show all work
please answer them correctly and show all work
For a particle in a one-dimensional box, E=n2h2/(8ma2) 1. Use the mass of the electron for m to calculate the two lowest energies of an electron in a one-dimensional box of length a=0.60nm(0.60109m or 6.0AA). me=9.109382911031kgh=6.6261034Js 2. What is the energy difference between the n=2 and n=1 levels? What wavelength of light could promote an electron from the n=1 level to the n=2 level? What range of the electromagnetic spectrum is this
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


