Question: please answer will rate Consider the following reaction in aqueous solution: 5Br(aq)+BrO3(aq)+6H+(aq)3Br2(aq)+3H2O(l)semantics If the rate of disappearance of Br(aq) at a particular moment during the
please answer will rate 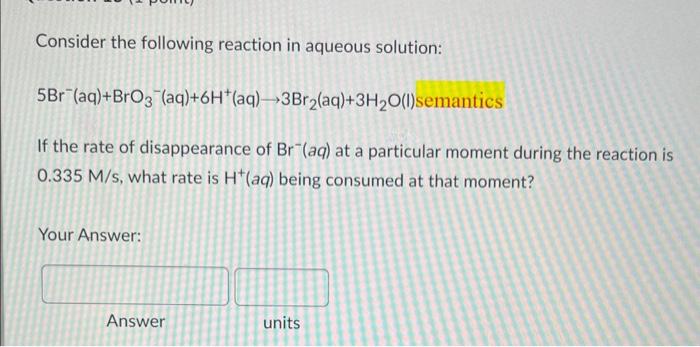

Consider the following reaction in aqueous solution: 5Br(aq)+BrO3(aq)+6H+(aq)3Br2(aq)+3H2O(l)semantics If the rate of disappearance of Br(aq) at a particular moment during the reaction is 0.335M/s, what rate is H+(aq) being consumed at that moment? Your Answer: Answer units
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


