Question: Please asap! Thank you! (1) This is a Numeric Entry question/It is worth 1 point/ You have unlimited attempts / There is no attempt penalty
Please asap! Thank you!
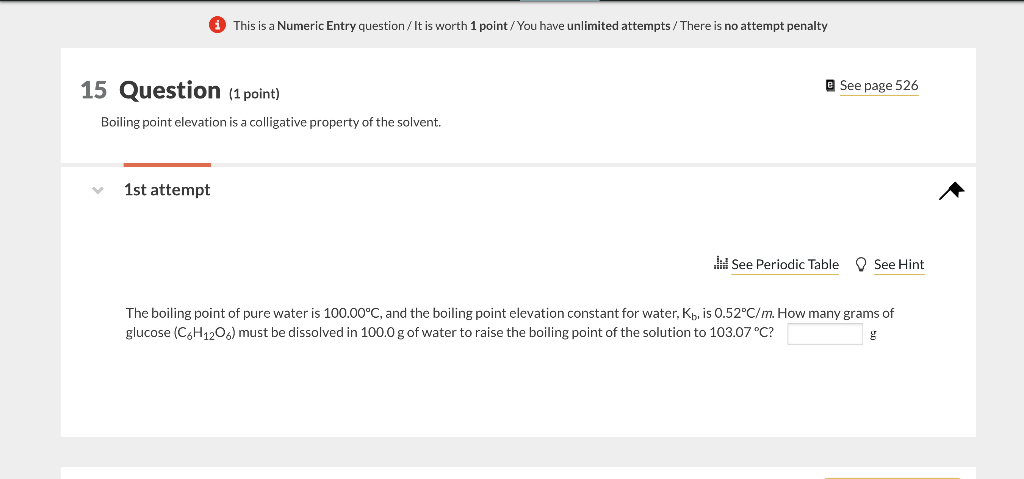
(1) This is a Numeric Entry question/It is worth 1 point/ You have unlimited attempts / There is no attempt penalty 15 Question (1 point) e See page 526 Boiling point elevation is a colligative property of the solvent. 1st attempt The boiling point of pure water is 100.00C, and the boiling point elevation constant for water, Kb, is 0.52C/m. How many grams of glucose (C6H12O6) must be dissolved in 100.0g of water to raise the boiling point of the solution to 103.07C ? g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


