Question: *Please be explicit in development and use Cengel tables. Thanks! Urea, a product of protein metabolism, is used pharmacologically parenterally and topically in the treatment
*Please be explicit in development and use Cengel tables. Thanks!
Urea, a product of protein metabolism, is used pharmacologically parenterally and topically in the treatment of various conditions. In the pharmaceutical industry, urea is synthesized by reacting ammonia and carbon dioxide to produce ammonium carbamate, which is then thermally decomposed into urea and water. The reaction system is as follows, represented at 25C
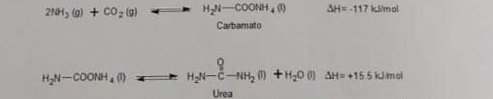
a) Determine the enthalpy change of urea formation at 25C __________ kJ/mol.
b) Mark with a strikeout. The synthesis of urea from the elements that make it up is: Exothermic Endothermic
2AH3(g)+CO2(9)H2NCOONH4(g)SH=117kJ/mol Carbamato H2NCOONH4(O)H2NCNH2(1)+H2ON(SH=+15.5kJmal
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


