Question: Please can I get a response quick Show ALL you work The following liquid based reaction takes place in batch unit. DE It is required
 Please can I get a response quick
Please can I get a response quick
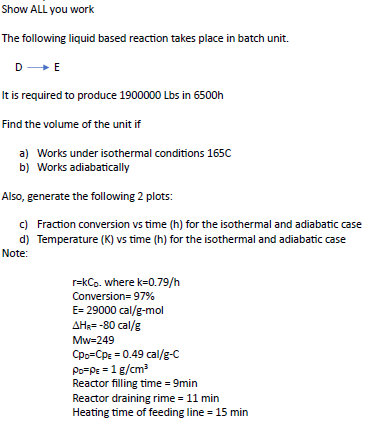
Show ALL you work The following liquid based reaction takes place in batch unit. DE It is required to produce 1900000Lbs in 6500h Find the volume of the unit if a) Works under isothermal conditions 165C b) Works adiabatically Also, generate the following 2 plots: c) Fraction conversion vs time ( h ) for the isothermal and adiabatic case d) Temperature ( K) vs time ( h ) for the isothermal and adiabatic case Note: r=kCD. where k=0.79/h Conversion =97% E=29000cal/gmol HR=80cal/g Mw=249 CD=CpE=0.49cal/gC 0=g=1g/cm3 Reactor filling time =9min Reactor draining rime =11min Heating time of feeding line =15min Show ALL you work The following liquid based reaction takes place in batch unit. DE It is required to produce 1900000Lbs in 6500h Find the volume of the unit if a) Works under isothermal conditions 165C b) Works adiabatically Also, generate the following 2 plots: c) Fraction conversion vs time ( h ) for the isothermal and adiabatic case d) Temperature ( K) vs time ( h ) for the isothermal and adiabatic case Note: r=kCD. where k=0.79/h Conversion =97% E=29000cal/gmol HR=80cal/g Mw=249 CD=CpE=0.49cal/gC 0=g=1g/cm3 Reactor filling time =9min Reactor draining rime =11min Heating time of feeding line =15min
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


