Question: Please consider the answers provided for the question and do not copy other chegg answers. 6.26 The heat of mixing data for water (1) and
Please consider the answers provided for the question and do not copy other chegg answers.
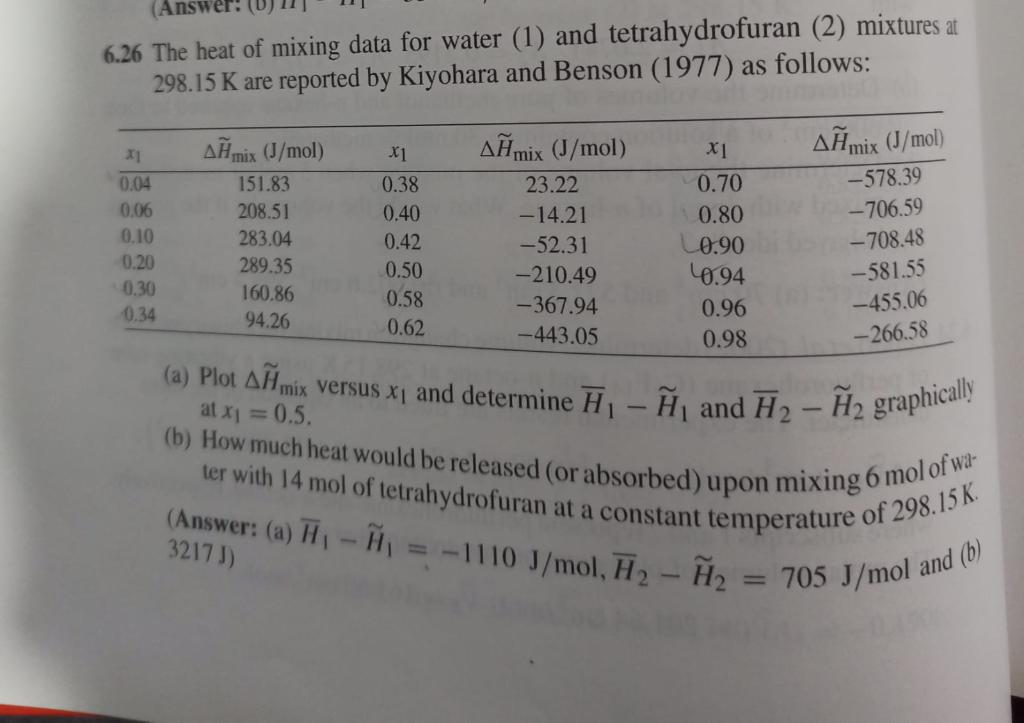
6.26 The heat of mixing data for water (1) and tetrahydrofuran (2) mixtures at 298.15K are reported by Kiyohara and Benson (1977) as follows: (a) Plot Hmix versus x1 and determine H1H1 and H2H2 graphically at x1=0.5 (b) How much heat would be released (or absorbed) upon mixing 6 mol of water with 14 mol of tetrahydrofuran at a constant temperature of 298.15K. (Answer: (a) H1H1=1110J/mol,H2H2=705J/mol and (b)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


