Question: Please, could you solve it as soon as possible I need it. 2. A first-order reaction; AB is to be carried out in two adiabatic
Please, could you solve it as soon as possible I need it.
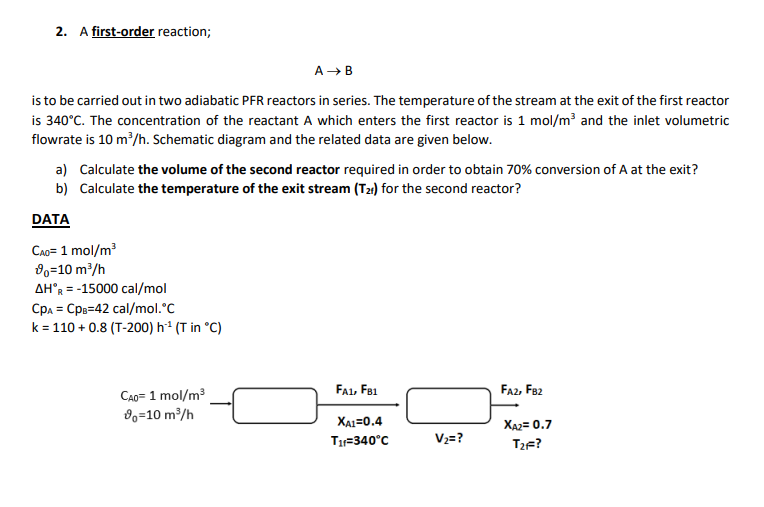
2. A first-order reaction; AB is to be carried out in two adiabatic PFR reactors in series. The temperature of the stream at the exit of the first reactor is 340C. The concentration of the reactant A which enters the first reactor is 1mol/m3 and the inlet volumetric flowrate is 10m3/h. Schematic diagram and the related data are given below. a) Calculate the volume of the second reactor required in order to obtain 70% conversion of A at the exit? b) Calculate the temperature of the exit stream (T2f) for the second reactor? DATA CA0=1mol/m30=10m3/hHR=15000cal/molCpA=CpB=42cal/mol.Ck=110+0.8(T200)h1(TinC) 2. A first-order reaction; AB is to be carried out in two adiabatic PFR reactors in series. The temperature of the stream at the exit of the first reactor is 340C. The concentration of the reactant A which enters the first reactor is 1mol/m3 and the inlet volumetric flowrate is 10m3/h. Schematic diagram and the related data are given below. a) Calculate the volume of the second reactor required in order to obtain 70% conversion of A at the exit? b) Calculate the temperature of the exit stream (T2f) for the second reactor? DATA CA0=1mol/m30=10m3/hHR=15000cal/molCpA=CpB=42cal/mol.Ck=110+0.8(T200)h1(TinC)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


