Question: please do both pleasee and thank u Write the equilibrium constant expression, K, for the following reaction: If either the numerator or denominator is blank,
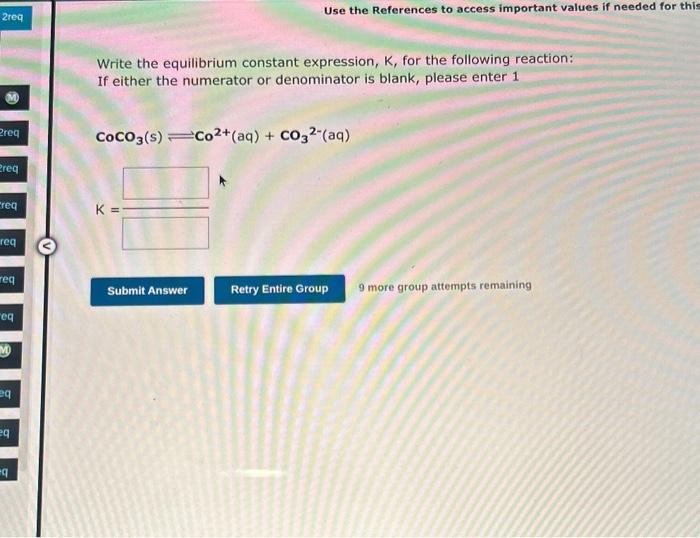

Write the equilibrium constant expression, K, for the following reaction: If either the numerator or denominator is blank, please enter 1 CoCO3(s)Co2+(aq)+CO32(aq) K= 9 more group attempts remaining At high temperatures, elemental nitrogen and oxygen react wath each other to form nitrogen monoxide: N1(g)+O2(g)=2NO(g) Suppose the system is analyzed at a particular temperature, and the equalbrium concentrations are found to be [N3]=0.037H3[O3]=0.085M,and[NO=5.0106,M1 Calculate the value of K for the reaction. k=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


