Question: i need help for the three questions Write the equilibrium constant expression, K, for the following reaction. Please enter the compounds in the order given
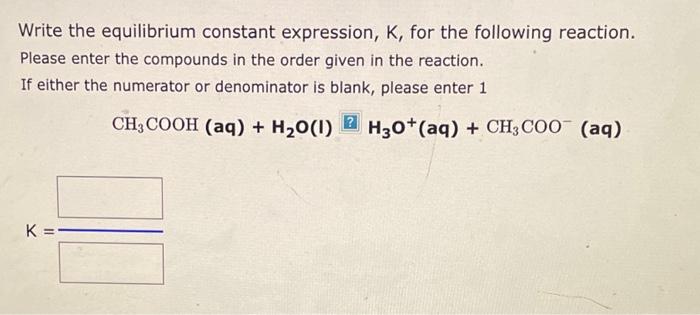

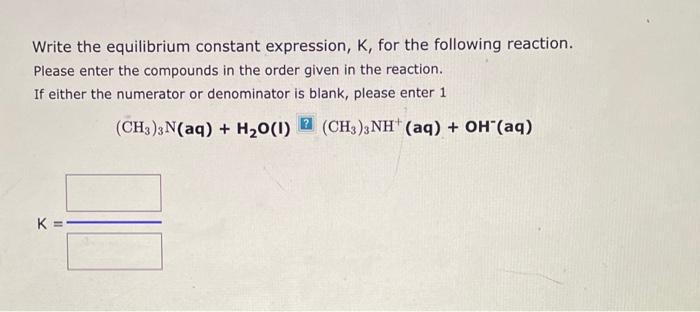
Write the equilibrium constant expression, K, for the following reaction. Please enter the compounds in the order given in the reaction. If either the numerator or denominator is blank, please enter 1 CH3COOH(aq)+H2O(I)H3O+(aq)+CH3COO(aq) Write the equilibrium constant expression, Kc, for the following reaction: (If either the numerator or denominator is 1, please enter 1.) 2Ag+(aq)+CO32(aq)Ag2CO3(s) Kc= Write the equilibrium constant expression, K, for the following reaction. Please enter the compounds in the order given in the reaction. If either the numerator or denominator is blank, please enter 1 (CH3)3N(aq)+H2O(I)[(CH3)3NH+(aq)+OH(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


