Question: please do both thank youu please Use the References to access important waluec if needed for this quastian. The equilibrium constant, Kc, for the following
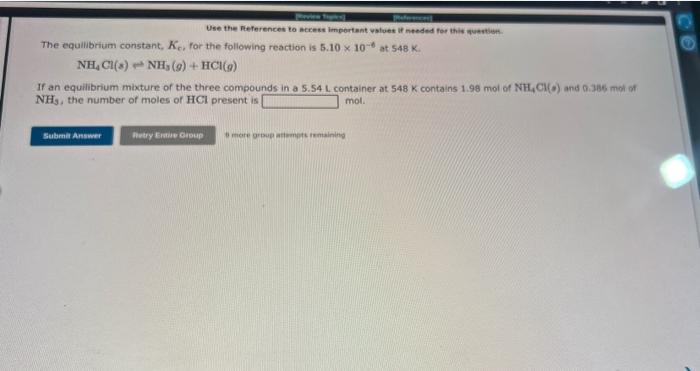
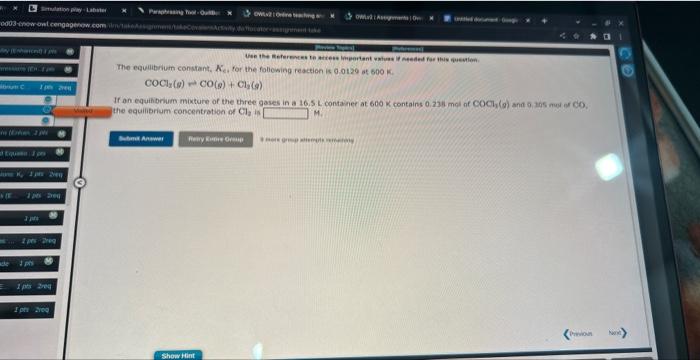
Use the References to access important waluec if needed for this quastian. The equilibrium constant, Kc, for the following reaction is 5.10106 at 548K. If an equilibrium mixture of the three compounds in a 5.54L container at 548K contains 1.98 mol of NH4Cl(o) and 0.386 mot of NH3, the number of moles of HCl present is mol. COClr()CO()+Cl2() If an equihbrium mixture of the three gases in a 16.5L contanef at 600K contains 0.23m mol nf COCl3(9) ant 0 , as nut of co. the equilibrium concentration of Cily in Mis
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


