Question: Use the References to access important values if needed for this question. The equilibrium constant, Kc for the following reaction is 1.77102 at 692K. 2HI(g)H2(g)+I2(g)
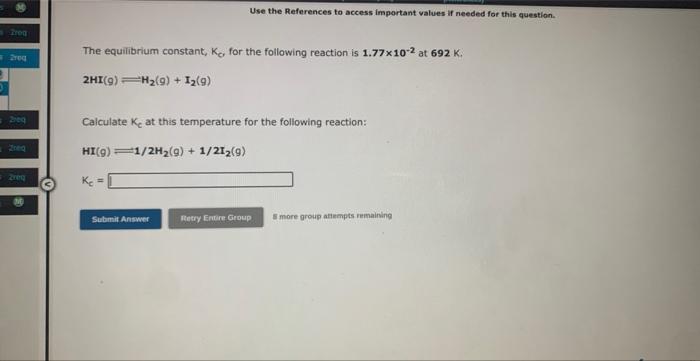
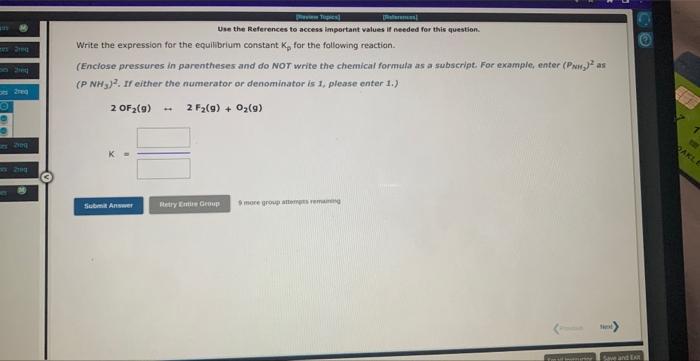
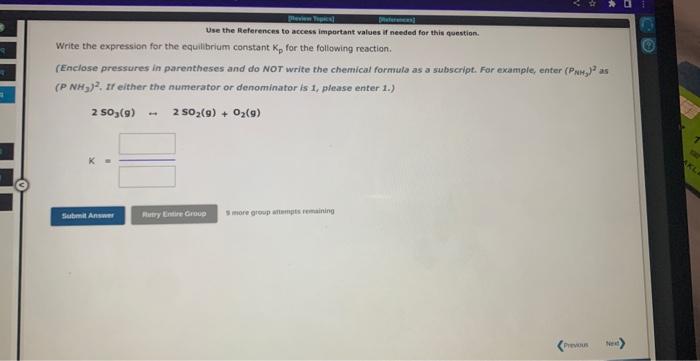
Use the References to access important values if needed for this question. The equilibrium constant, Kc for the following reaction is 1.77102 at 692K. 2HI(g)H2(g)+I2(g) Calculate Kc at this temperature for the following reaction: HI(g)1/2H2(g)+1/2I2(g)Kc=1 Use the References to access important values if needed for this question. Write the expression for the equilibrium constant. Kp for the following reaction. (Enclose pressures in parentheses and do NoT write the chemical formula as as subscript. For example, enter (Ping) as (PNH3)2. If either the numerator or denominator is 1, please enter 1.). 2OF2(g)2F2(g)+O2(g) Use the References to access important values if needed for this question. Write the expression for the equilibrium constant Kp for the following reaction. (Enclose pressures in parentheses and do NOT write the chemical formala as a subseript. Far example, enter (PNM, J as (PNH3)2, if either the numerator or denominator is 1 , please enter 1.) 2503(9)2502(9)+02(9) 5 more atesp atienpts restaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


