Question: please do bothh thank uu Use the References to access important values if needed for this question. The reaction of nitrogen monoxide with ozone at
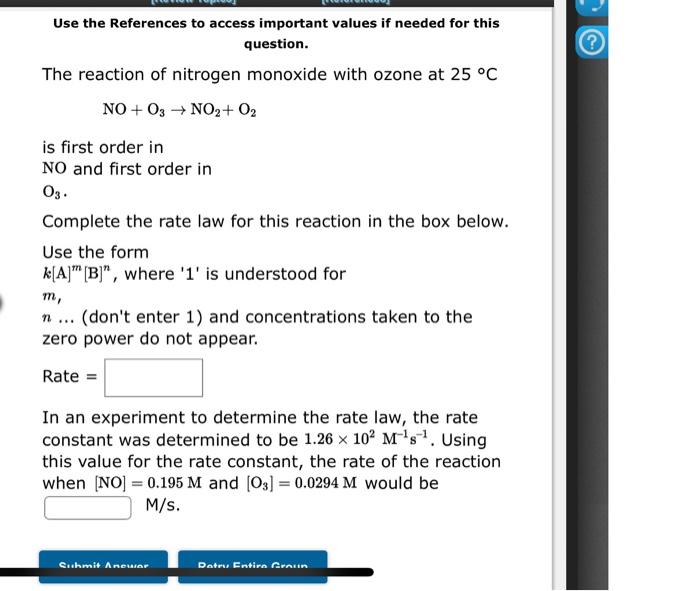
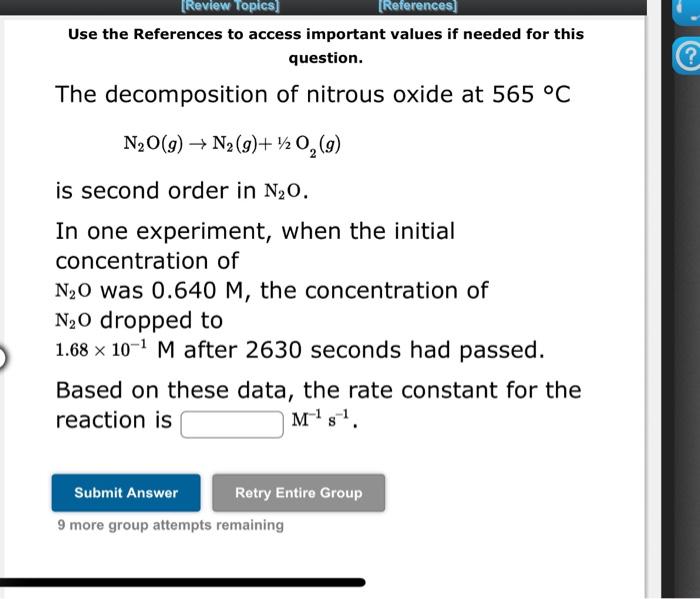
Use the References to access important values if needed for this question. The reaction of nitrogen monoxide with ozone at 25C NO+O3NO2+O2 is first order in NO and first order in O3. Complete the rate law for this reaction in the box below. Use the form k[A]m[B]n, where ' 1 ' is understood for m, n... (don't enter 1 ) and concentrations taken to the zero power do not appear. Rate = In an experiment to determine the rate law, the rate constant was determined to be 1.26102M1s1. Using this value for the rate constant, the rate of the reaction when [NO]=0.195M and [O3]=0.0294M would be M/s. Use the References to access important values if needed for this question. The decomposition of nitrous oxide at 565C N2O(g)N2(g)+1/2O2(g) is second order in N2O. In one experiment, when the initial concentration of N2O was 0.640M, the concentration of N2O dropped to 1.68101M after 2630 seconds had passed. Based on these data, the rate constant for the reaction is M1s1 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


