Question: Use the References to access important values if needed for this question. The reaction of nitrogen dioxide with fluorine 2NO2+F22NO2F is first order in NO2
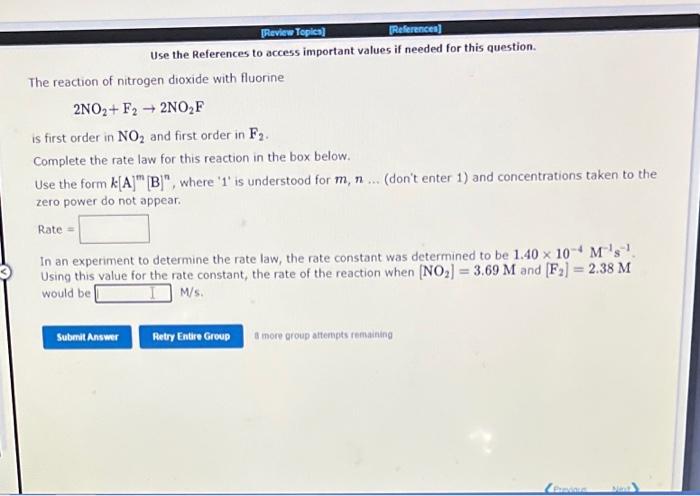
Use the References to access important values if needed for this question. The reaction of nitrogen dioxide with fluorine 2NO2+F22NO2F is first order in NO2 and first order in F2. Complete the rate law for this reaction in the box below. Use the form k[A]m[B]n, where ' 1 ' is understood for m,n (don't enter 1 ) and concentrations taken to the zero power do not appear. Rate = In an experiment to determine the rate law, the rate constant was determined to be 1.40104M1s1 Using this value for the rate constant, the rate of the reaction when [NO2]=3.69M and [F2]=2.38M would be M/s.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


