Question: Please DO NOT COPY from other answer. That answer is completely wrong. A dimerization reaction of 2R molecules occurs on a catalyst surface to form
Please DO NOT COPY from other answer. That answer is completely wrong.
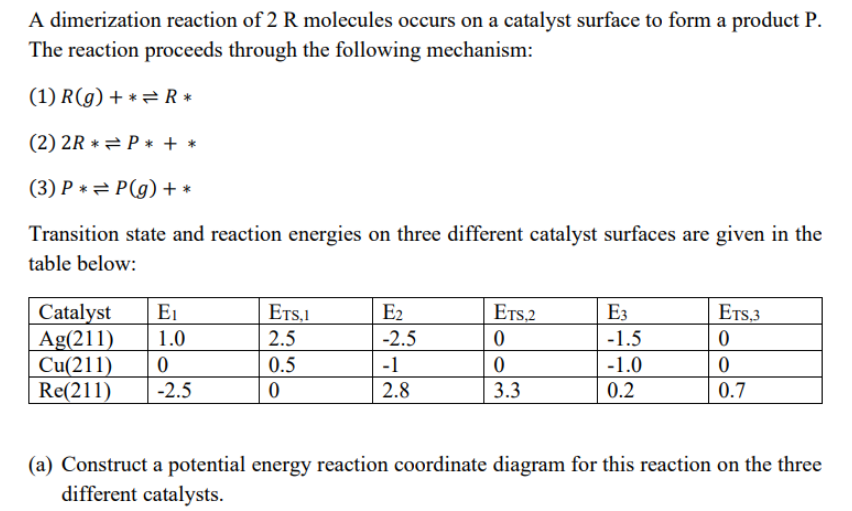
A dimerization reaction of 2R molecules occurs on a catalyst surface to form a product P. The reaction proceeds through the following mechanism: (1) R(g)+R (2) 2RP+ (3) PP(g)+ Transition state and reaction energies on three different catalyst surfaces are given in the table below: (a) Construct a potential energy reaction coordinate diagram for this reaction on the three different catalysts
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


