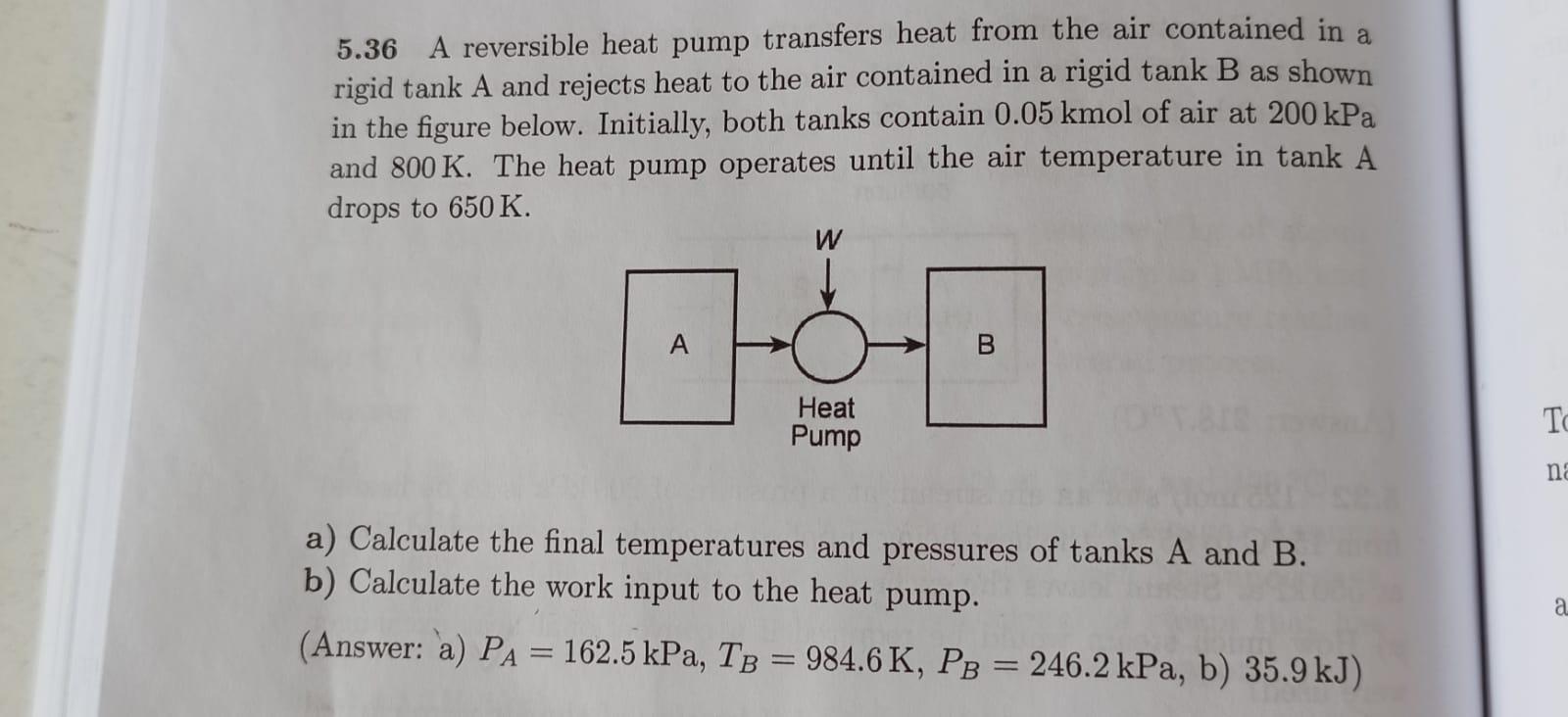
Question: PLEASE, DO NOT TAKE OTHER CHEGG SOLUTION, BECAUSE IT IS NOT CLEAR 5.36 A reversible heat pump transfers heat from the air contained in a
 PLEASE, DO NOT TAKE OTHER CHEGG SOLUTION, BECAUSE IT IS NOT CLEAR
PLEASE, DO NOT TAKE OTHER CHEGG SOLUTION, BECAUSE IT IS NOT CLEAR
5.36 A reversible heat pump transfers heat from the air contained in a rigid tank A and rejects heat to the air contained in a rigid tank B as shown in the figure below. Initially, both tanks contain 0.05kmol of air at 200kPa and 800K. The heat pump operates until the air temperature in tank A drops to 650K. a) Calculate the final temperatures and pressures of tanks A and B. b) Calculate the work input to the heat pump. (Answer: a) PA=162.5kPa,TB=984.6K,PB=246.2kPa,b)35.9kJ )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


