Question: PLEASE DO ONLY B!!! Calcium hydroxide dissolves into water as Ca2+ and OH ions. The solubility of calcium hydroxide, Ca(OH)2, at 25C was measured to
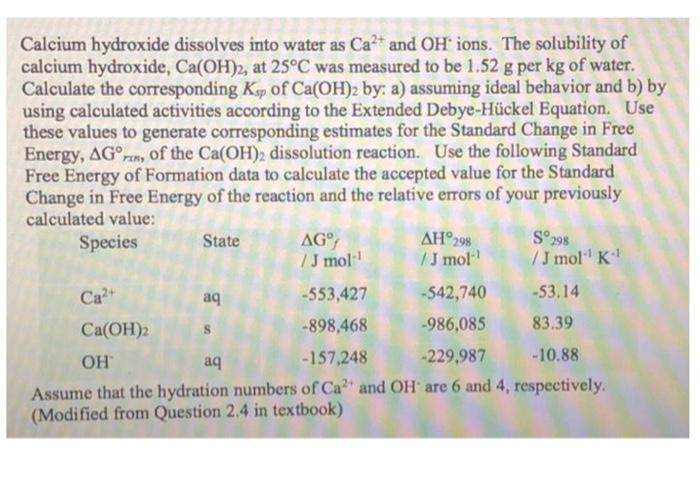
Calcium hydroxide dissolves into water as Ca2+ and OH ions. The solubility of calcium hydroxide, Ca(OH)2, at 25C was measured to be 1.52 g per kg of water. Calculate the corresponding Ksp of Ca(OH)2 by: a) assuming ideal behavior and b) by using calculated activities according to the Extended Debye-Hckel Equation. Use these values to generate corresponding estimates for the Standard Change in Free Energy, AGren, of the Ca(OH)2 dissolution reaction. Use the following Standard Free Energy of Formation data to calculate the accepted value for the Standard Change in Free Energy of the reaction and the relative errors of your previously calculated value: Species State AG AH298 S298 /J mol /J mol /Jmol 'K Ca2+ -553,427 -542,740 -53.14 Ca(OH)2 s -898,468 -986,085 83.39 OH aq - 157,248 -229,987 -10.88 Assume that the hydration numbers of Ca+ and OH are 6 and 4, respectively. (Modified from Question 2.4 in textbook) a
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


