Question: PLEASE DO PART D ONLY!!! Consider the liquid reaction: Ca=0.01Cao AB is to be carried out isothermally in a continuous flow reactor. Calculate both the
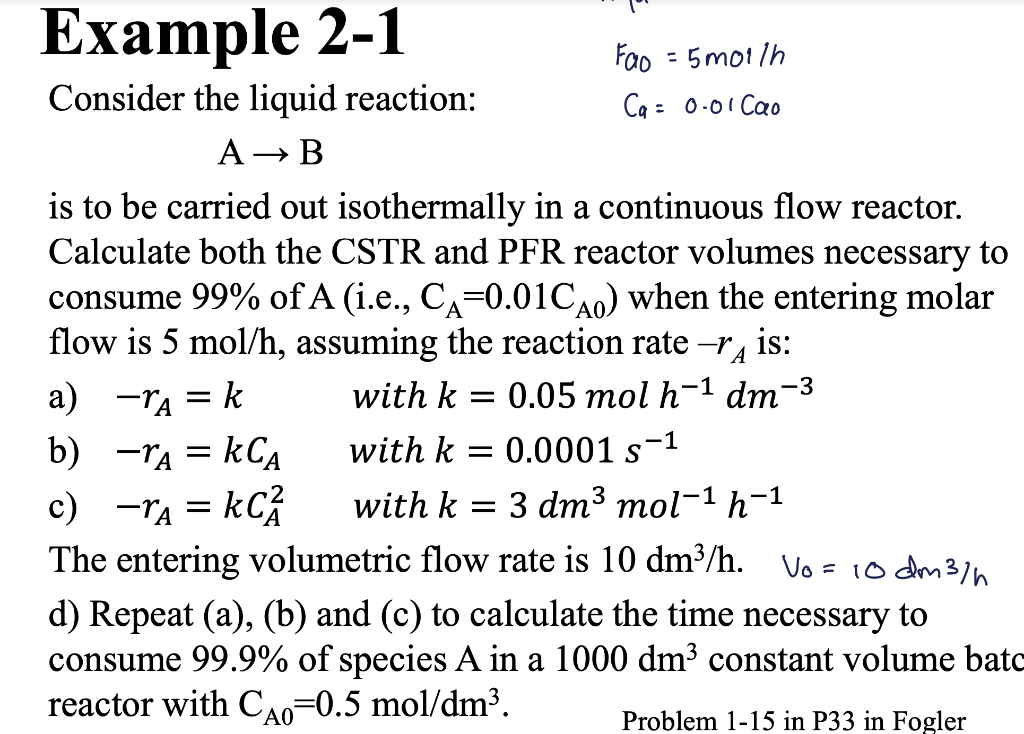
PLEASE DO PART D ONLY!!!
Consider the liquid reaction: Ca=0.01Cao AB is to be carried out isothermally in a continuous flow reactor. Calculate both the CSTR and PFR reactor volumes necessary to consume 99% of A (i.e., CA=0.01CA0 ) when the entering molar flow is 5mol/h, assuming the reaction rate rA is: a) rA=k with k=0.05molh1dm3 b) rA=kCA with k=0.0001s1 c) rA=kCA2 with k=3dm3mol1h1 The entering volumetric flow rate is 10dm3/h.V0=10dm3/h d) Repeat (a), (b) and (c) to calculate the time necessary to consume 99.9% of species A in a 1000dm3 constant volume ba reactor with CA0=0.5mol/dm3. Problem 115 in P33 in Fogler
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


