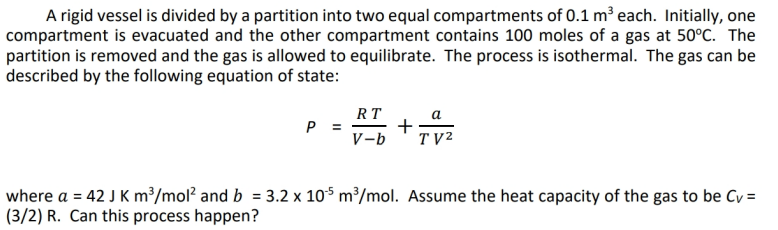
Question: Please do your own work. A rigid vessel is divided by a partition into two equal compartments of 0.1 m'each. Initially, one compartment is evacuated
Please do your own work.
A rigid vessel is divided by a partition into two equal compartments of 0.1 m'each. Initially, one compartment is evacuated and the other compartment contains 100 moles of a gas at 50C. The partition is removed and the gas is allowed to equilibrate. The process is isothermal. The gas can be described by the following equation of state: RT a P = - + V-b TV2 where a = 42 J K m3/mol and b = 3.2 x 10-5 m?/mol. Assume the heat capacity of the gas to be Cv = (3/2) R. Can this process happen
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


