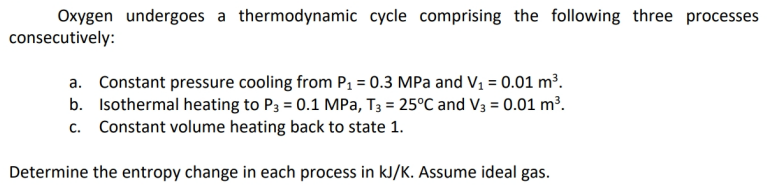
Question: Please do your own work Oxygen undergoes a thermodynamic cycle comprising the following three processes consecutively: a. Constant pressure cooling from P1 = 0.3 MPa
Please do your own work
Oxygen undergoes a thermodynamic cycle comprising the following three processes consecutively: a. Constant pressure cooling from P1 = 0.3 MPa and V1 = 0.01 m. b. Isothermal heating to P3 = 0.1 MPa, T3 = 25C and V3 = 0.01 m. C. Constant volume heating back to state 1. Determine the entropy change in each process in kJ/K. Assume ideal gas
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


