Question: please dont answer if you are not going to cleary draw and show all work and steps no shortcuts please 1. A lake with a
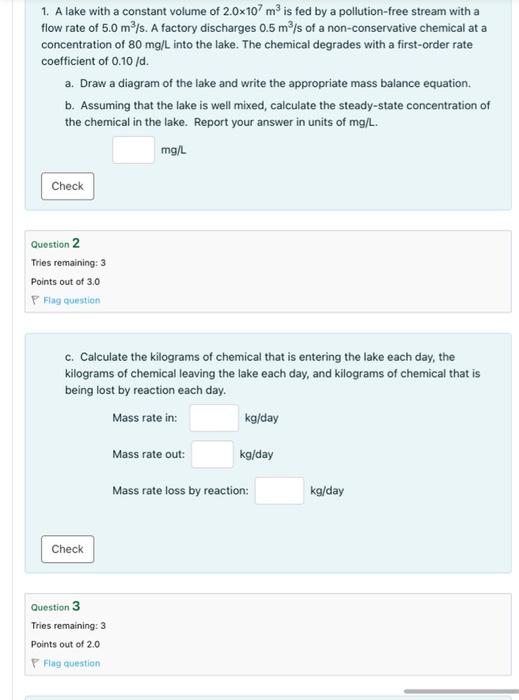
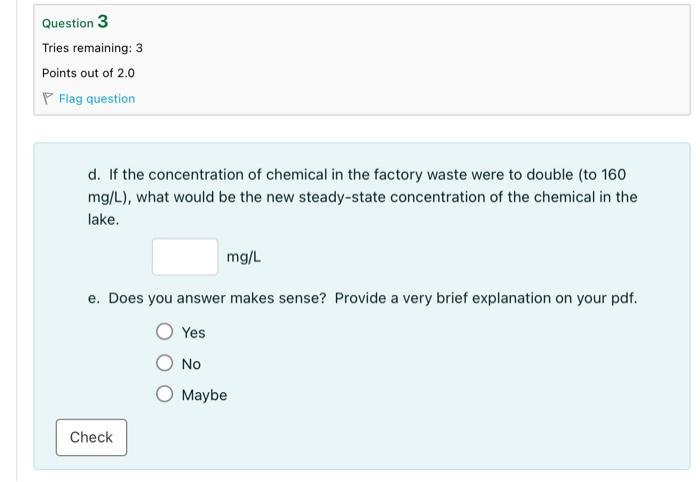
1. A lake with a constant volume of 2.0107m3 is fed by a pollution-free stream with a flow rate of 5.0m3/s. A factory discharges 0.5m3/s of a non-conservative chemical at a concentration of 80mg/L into the lake. The chemical degrades with a first-order rate coefficient of 0.10/d. a. Draw a diagram of the lake and write the appropriate mass balance equation. b. Assuming that the lake is well mixed, calculate the steady-state concentration of the chemical in the lake. Report your answer in units of mg/L. Question 2 Tries remaining: 3 Points out of 3.0 P Flag question c. Calculate the kilograms of chemical that is entering the lake each day, the kilograms of chemical leaving the lake each day, and kilograms of chemical that is being lost by reaction each day. Mass rate in: kg/day Mass rate out: kg/day Mass rate loss by reaction: kg/day d. If the concentration of chemical in the factory waste were to double (to 160 mg/L ), what would be the new steady-state concentration of the chemical in the lake. e. Does you answer makes sense? Provide a very brief explanation on your pdf. Yes No Maybe
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


