Question: 6) HA is a weak acid. Which equilibrium corresponds to the equilibrium constant Kb for A-? A) A- (aq) + HO (1) HA (aq)
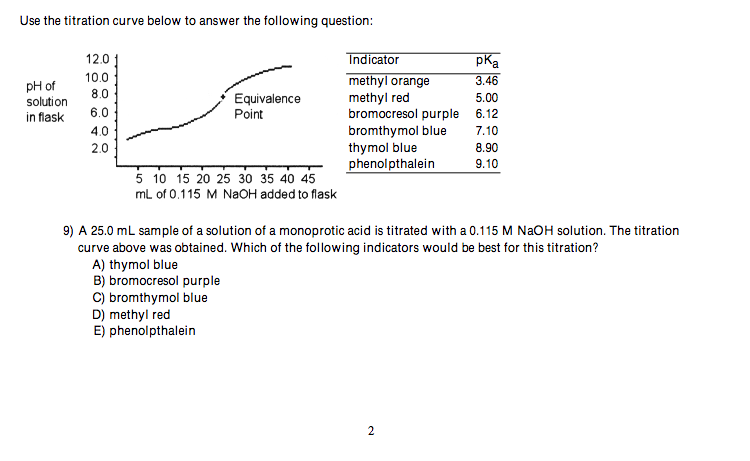
6) HA is a weak acid. Which equilibrium corresponds to the equilibrium constant Kb for A-? A) A- (aq) + HO (1) HA (aq) + OH- (aq) HOA2- (aq) B) A (aq) + OH- (aq) HA+ (aq) + OH- (aq) C) HA (aq) + HO (1) D) HA (aq) + OH- (aq) H0 (1) + H+ (aq) E) A- (aq) + H3O+ (aq) HA (aq) + HO (1) 7) A Brnsted-Lowry base is defined as a substance that A) acts as a proton acceptor B) decreases [H+] when placed in HO C) increases [OH-] when placed in HO D) increases [H+] when placed in HO E) acts as a proton donor 8) What are the Brnsted-Lowry bases in the following chemical reaction? A) C5 H5N, OH- C) C5 H5N, C5H5NH+ C5 H5N(aq) + HO(1) C5H5NH+(aq) + OH- (aq) B) C5H5N, H2O, OH- D) C5H5N, H2O Use the titration curve below to answer the following question: pH of solution in flask 12.0 10.0 8.0 6.0 4.0 2.0 Equivalence Point 5 10 15 20 25 30 35 40 45 mL of 0.115 M NaOH added to flask Indicator methyl orange methyl red bromocresol purple bromthymol blue thymol blue phenolpthalein pka 3.46 2 5.00 6.12 7.10 8.90 9.10 9) A 25.0 mL sample of a solution of a monoprotic acid is titrated with a 0.115 M NaOH solution. The titration curve above was obtained. Which of the following indicators would be best for this titration? A) thymol blue B) bromocresol purple C) bromthymol blue D) methyl red E) phenolpthalein
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
6 a 7 a 8 ... View full answer

Get step-by-step solutions from verified subject matter experts


