Question: please explain how to derive mechanism form main equation and rate data b) Understanding of oxidative desulphurization kinetics is important if there is ever a
please explain how to derive mechanism form main equation and rate data
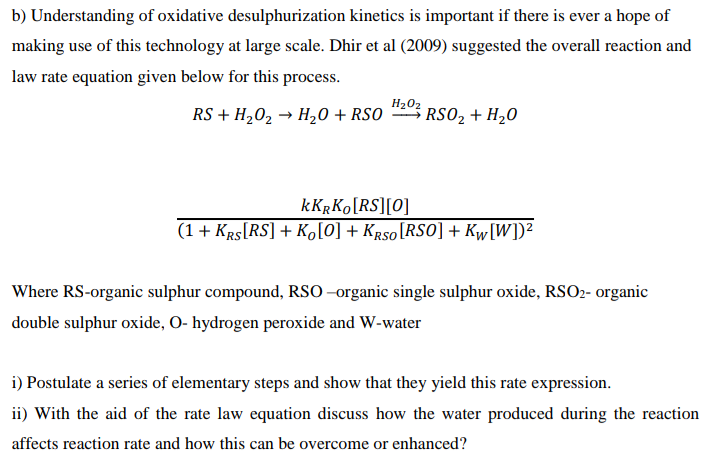
b) Understanding of oxidative desulphurization kinetics is important if there is ever a hope of making use of this technology at large scale. Dhir et al (2009) suggested the overall reaction and law rate equation given below for this process. RS + H202 + H20 + RS0 H2O3 RS02 + H20 kKRKO[RS][O] (1 + KRS[RS] + K [0] + KRS[RSO] + Kw[W])2 Where RS-organic sulphur compound, RSO-organic single sulphur oxide, RSO2- organic double sulphur oxide, O- hydrogen peroxide and W-water i) Postulate a series of elementary steps and show that they yield this rate expression. ii) With the aid of the rate law equation discuss how the water produced during the reaction affects reaction rate and how this can be overcome or enhanced? b) Understanding of oxidative desulphurization kinetics is important if there is ever a hope of making use of this technology at large scale. Dhir et al (2009) suggested the overall reaction and law rate equation given below for this process. RS + H202 + H20 + RS0 H2O3 RS02 + H20 kKRKO[RS][O] (1 + KRS[RS] + K [0] + KRS[RSO] + Kw[W])2 Where RS-organic sulphur compound, RSO-organic single sulphur oxide, RSO2- organic double sulphur oxide, O- hydrogen peroxide and W-water i) Postulate a series of elementary steps and show that they yield this rate expression. ii) With the aid of the rate law equation discuss how the water produced during the reaction affects reaction rate and how this can be overcome or enhanced
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


