Question: please explain step by step how to solve part c and d. i dont need a and b. 6. Consider the following unbalanced reaction: B2H6(s)+O2(s)H3B3O(5)+H2O(1)
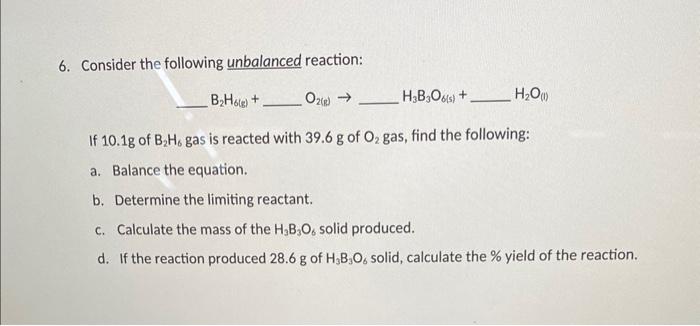
6. Consider the following unbalanced reaction: B2H6(s)+O2(s)H3B3O(5)+H2O(1) If 10.1g of B2H6 gas is reacted with 39.6g of O2 gas, find the following: a. Balance the equation. b. Determine the limiting reactant. c. Calculate the mass of the H3B3O6 solid produced. d. If the reaction produced 28.6g of H3B3O6 solid, calculate the % yield of the reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


