Question: Please explain while solving the question Question 1 2 pts At pH 7.35, determine the concentration of H3PO4 and PO43 and check for yourself why
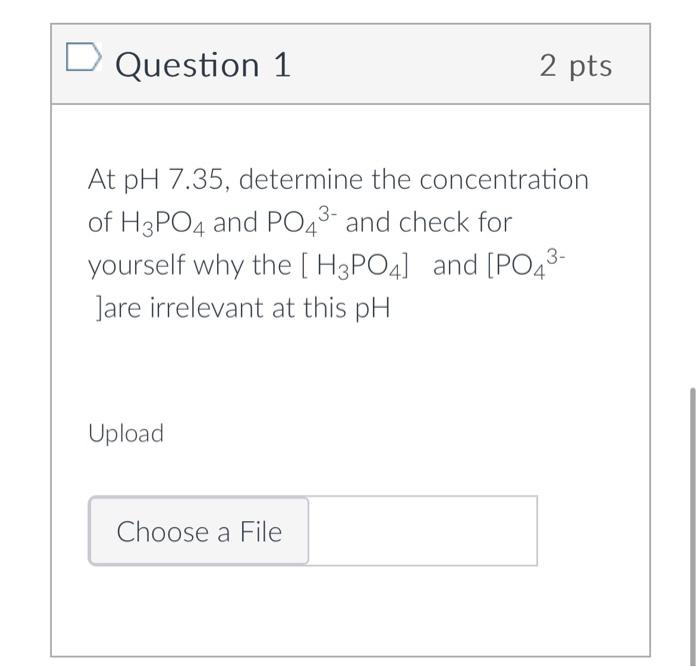
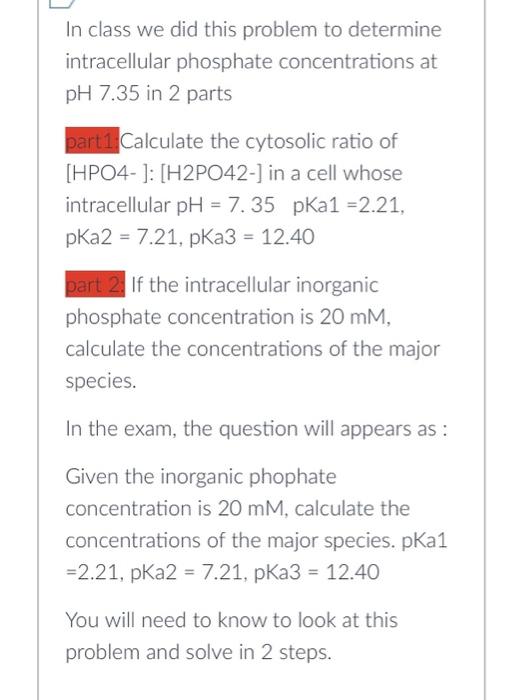
Question 1 2 pts At pH 7.35, determine the concentration of H3PO4 and PO43 and check for yourself why the [H3PO4] and [PO43 Jare irrelevant at this pH Upload In class we did this problem to determine intracellular phosphate concentrations at pH7.35 in 2 parts part1.Calculate the cytosolic ratio of [HPO4- ]: [H2PO42-] in a cell whose intracellular pH=7.35 pKa1 =2.21, pKa2=7.21,pKa3=12.40 part 2. If the intracellular inorganic phosphate concentration is 20mM, calculate the concentrations of the major species. In the exam, the question will appears as: Given the inorganic phophate concentration is 20mM, calculate the concentrations of the major species. pKa1 =2.21,pKa2=7.21,pKa3=12.40 You will need to know to look at this problem and solve in 2 steps
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


