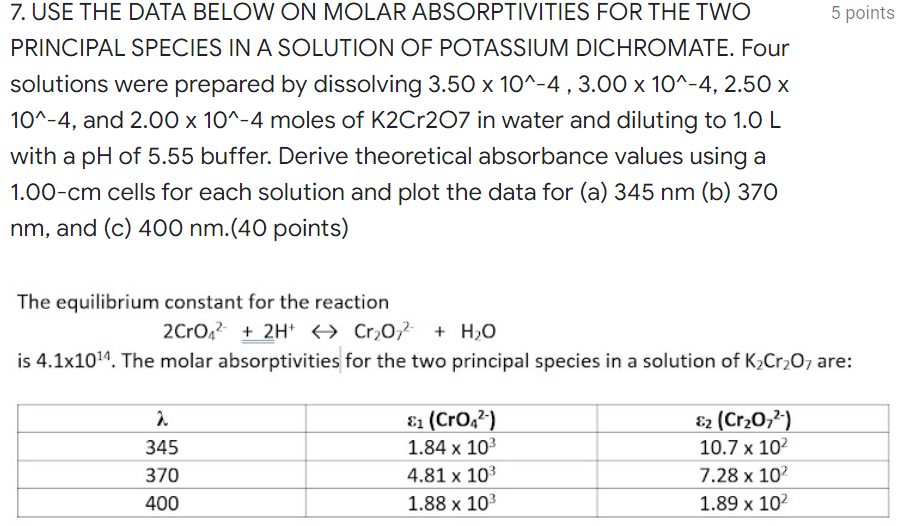
Question: Please help and show complete solutions. 5 points 7. USE THE DATA BELOW ON MOLAR ABSORPTIVITIES FOR THE TWO PRINCIPAL SPECIES IN A SOLUTION OF
Please help and show complete solutions.
5 points 7. USE THE DATA BELOW ON MOLAR ABSORPTIVITIES FOR THE TWO PRINCIPAL SPECIES IN A SOLUTION OF POTASSIUM DICHROMATE. Four solutions were prepared by dissolving 3.50 x 10^-4 , 3.00 x 10^-4, 2.50 x 10^-4, and 2.00 x 10^-4 moles of K2Cr207 in water and diluting to 1.0 L with a pH of 5.55 buffer. Derive theoretical absorbance values using a 1.00-cm cells for each solution and plot the data for (a) 345 nm (b) 370 nm, and (c) 400 nm.(40 points) The equilibrium constant for the reaction 2 CrO2 + 2H+ + Cr2O72- H + H2O is 4.1x1024. The molar absorptivities for the two principal species in a solution of K Cr2O7 are: 2. 345 370 400 &1 (CrO,?-) 1.84 x 10 4.81 x 103 1.88 x 103 E2 (Cr20,?) 10.7 x 102 7.28 x 102 1.89 x 102
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


