Question: please help correct For the reaction: 2HI(g)H2(g)+I2(g)Keq=0.016 Initially a container contains 0.37M of Hl and no product. What is the equilibrium concentration of H2
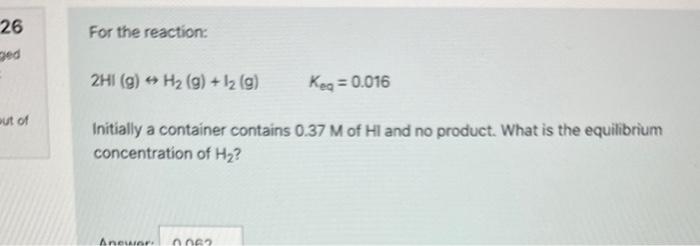
For the reaction: 2HI(g)H2(g)+I2(g)Keq=0.016 Initially a container contains 0.37M of Hl and no product. What is the equilibrium concentration of H2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


