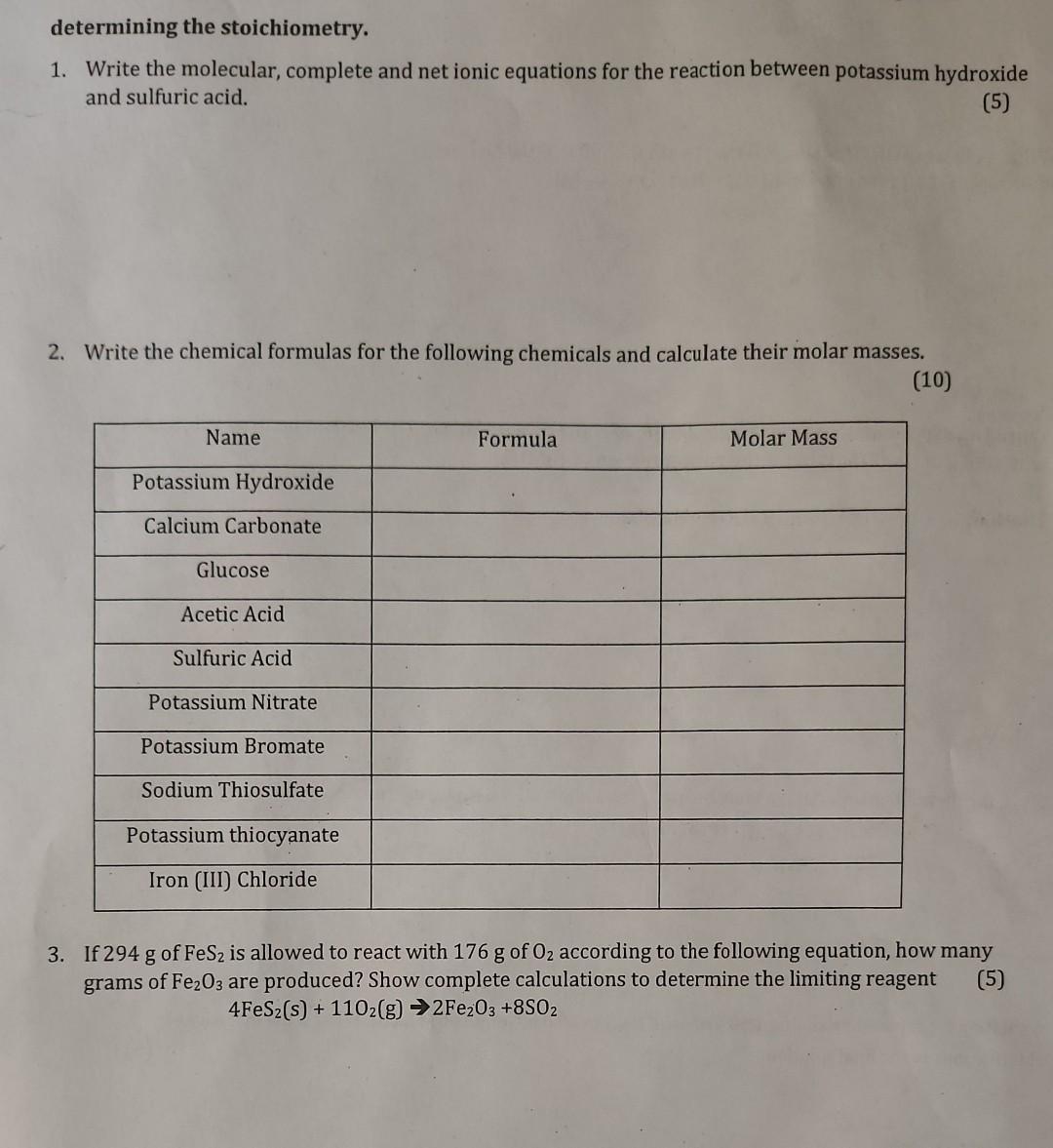
Question: please help determining the stoichiometry. 1. Write the molecular, complete and net ionic equations for the reaction between potassium hydroxide and sulfuric acid. (5) 2.
please help
determining the stoichiometry. 1. Write the molecular, complete and net ionic equations for the reaction between potassium hydroxide and sulfuric acid. (5) 2. Write the chemical formulas for the following chemicals and calculate their molar masses. (10) 3. If 294g of FeS2 is allowed to react with 176g of O2 according to the following equation, how many grams of Fe2O3 are produced? Show complete calculations to determine the limiting reagent (5) 4FeS2(s)+11O2(g)2Fe2O3+8SO2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


