Question: please help!! i included an example in the third picture i need help finding the info from the first pic This compound is a liquid
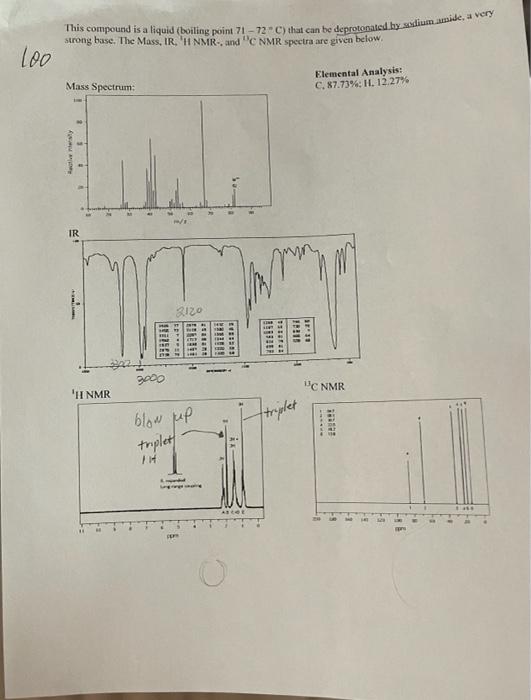

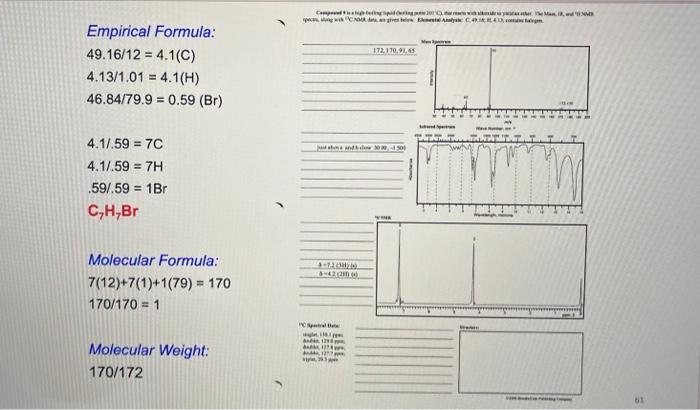
This compound is a liquid (boiling point 71 - 72C) that can be deprotonated by diuinide, a very strong base. The Mass, IR, H NMR. and C NMR spectra are given below. loo Mass Spectrum Elemental Analysis: C. 87.73% 11.12.27% actory IR AMA 2120 22 2000 WC NMR 'H NMR blow up triplet triplet IH OR Lab Report From your unknown data, report the following: 1. Elemental composition and calculation of the empirical formula. 2. Molecular (weight) mass-using the molecular ion peak in the MS. 3. Molecular formula. 4. Functional groups present based on IR peaks. 5. Number of different types of carbons based on 13C-NMR. 6. From the 'H-NMR spectrum, report the number of signals, their chemical shifts, integration splitting, and number of neighboring protons. . Determine the structure of your unknown compound using all the pieces of information from your spectral data sheet. Record unknown number on the data sheet and submit pages 82-83 for the report. mmn 172, 170,91,65 Empirical Formula: 49.16/12 = 4.1(C) 4.13/1.01 = 4.1(H) 46.84179.9 = 0.59 (Br) Mind 4.1/.59 = 7C 4.1/.59 = 7H .597.59 = 1Br C,H,Br Molecular Formula: 7(12)+7(1)+1(79) = 170 170/170 = 1 Molecular Weight: 170/172 51
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


