Question: please help!! i will thumbs up if correct! A researcher prepares a buffer of acetic acid and sodium acetate with a pH of 5.0. The
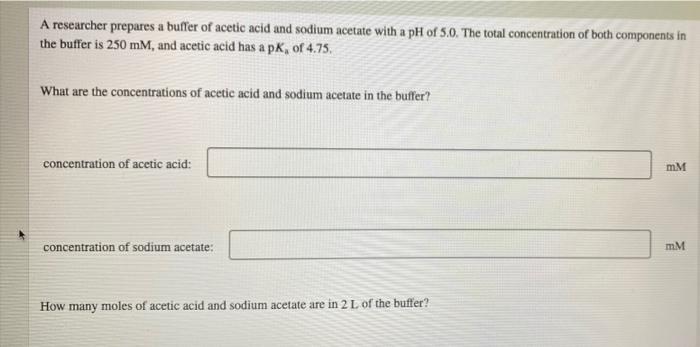
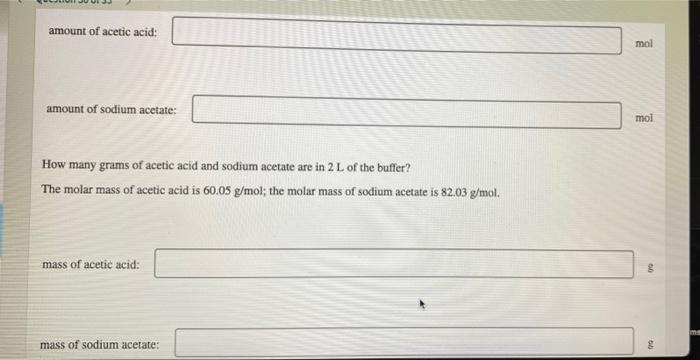
A researcher prepares a buffer of acetic acid and sodium acetate with a pH of 5.0. The total concentration of both components in the buffer is 250 mM, and acetic acid has a pK, of 4.75. What are the concentrations of acetic acid and sodium acetate in the buffer? concentration of acetic acid: mM concentration of sodium acetate: mM How many moles of acetic acid and sodium acetate are in 2 L of the buffer? amount of acetic acid: mol amount of sodium acetate; mol How many grams of acetic acid and sodium acetate are in 2 L of the buffer? The molar mass of acetic acid is 60.05 g/mol; the molar mass of sodium acetate is 82.03 g/mol. mass of acetic acid: mass of sodium acetate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


