Question: please help im really stuck A solution is 0.0100M in each of the metal ions in the followinn tahlo. HCl is added to the solution
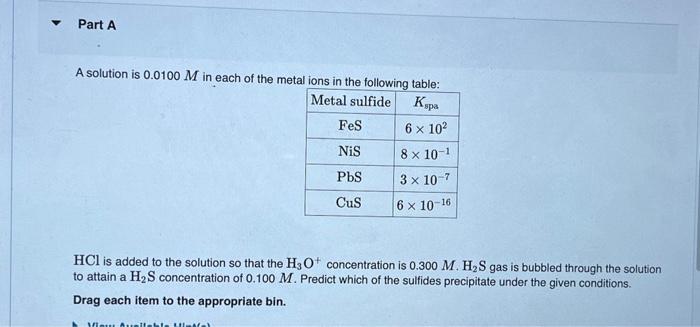

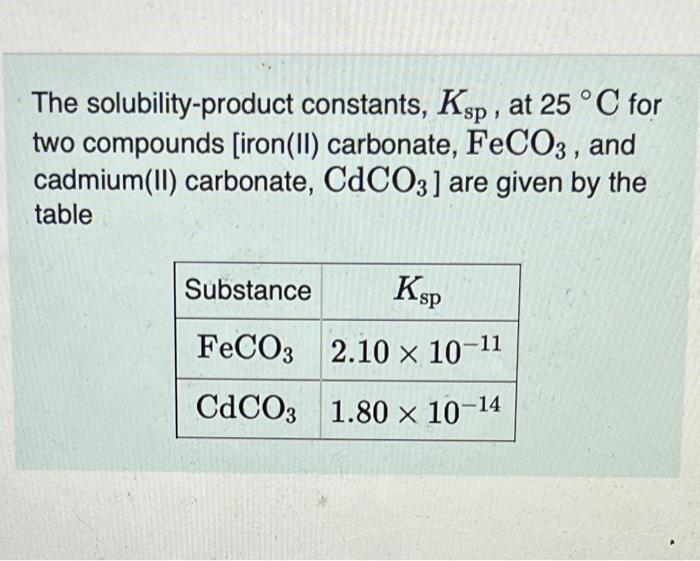
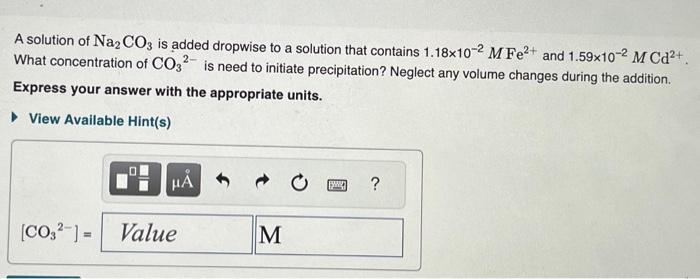
A solution is 0.0100M in each of the metal ions in the followinn tahlo. HCl is added to the solution so that the H3O+concentration is 0.300M.H2S gas is bubbled through the solution to attain a H2S concentration of 0.100M. Predict which of the sulfides precipitate under the given conditions. Drag each item to the appropriate bin. FeS NiS CuS PbS Will precipitate Will not precipitate The solubility-product constants, Ksp, at 25C for two compounds [iron(II) carbonate, FeCO3, and cadmium(II) carbonate, CdCO3 ] are given by the table A solution of Na2CO3 is added dropwise to a solution that contains 1.18102MFe2+ and 1.59102MCd2+. What concentration of CO32 is need to initiate precipitation? Neglect any volume changes during the addition. Express your answer with the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


