Question: please help! less than 24 hours left! here is the addition information! thank you! 1. Using the calibration graph and the scale readings for hydrogen,


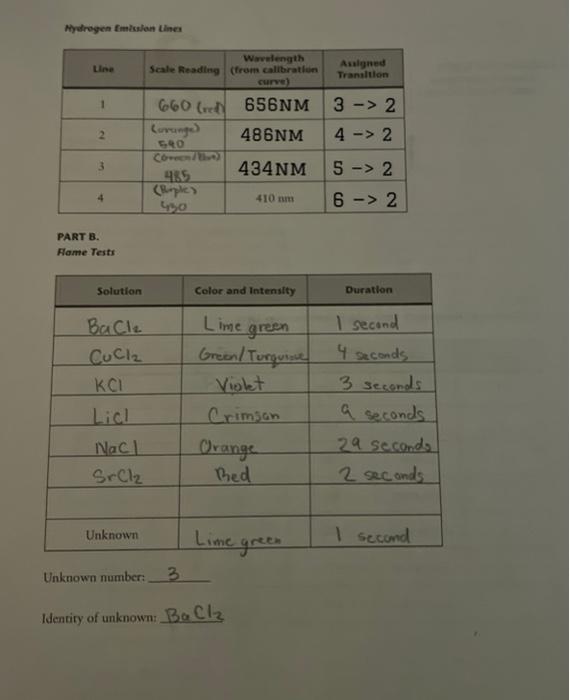
1. Using the calibration graph and the scale readings for hydrogen, determine the wavelengths for hydrogen emission; enter the results on your data sheet. 2. The four hydrogen lines correspond to the 32,42,52, and 62 transitions. Assign a transition to each wavelength and enter the results on your data sheet. Hint: The lowest wavelength, 410nm, corresponds to the highest-energy transition. The 32 transition emits the least energy. 3. Determine the Rydberg constant from the data. Since for n=2 : 1=RH(ni21nh21)1=22RHRH(nb21)1=RH(nb21)+4RH Equation 1 which is of the form y=mx+b That is, Equation 2 is of the form of a straight line; hence the Rydberg constant is the negative of the slope. Plot y=1/ vs. x=1b2 (in Excel or Google Sheets), then determine the slope m=RH. Be sure to label the axes of the graph. As a check, compare this value of RH with the y-intercept, which should equal RH/4. To make it easy, set up a table (next page) for the data. Use meters for wavelength; the units of RH will be meter 1. Show your calculations in the space below: 4. Calculate the change in energy for each transition, nb=3,4,5,6 and nc=2. Show your work and give the results in units of joules. E=bc=bcRH(n21nh21)Equation332:E=42:E=52:E=62:E= 5. Derive Equation 3 from Equation 1: 6. Determine the ionization energy for the hydrogen atom. The ionization energy is the energy necessary to take an clectron from its most stable state ( n=1 for hydrogen), to a state infinitely far from the nucleus. Use Equation 3 to make the calculation. What is nb ? Show your work. Mywhogen fimissian tines PART B. Fame Tests Unknown number: 3 Identiry of unknown: BaCl2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


