Question: please, help me. 1. Adding a solute to a solvent __ the freezing point because the chemical forces between the makes it harder for the

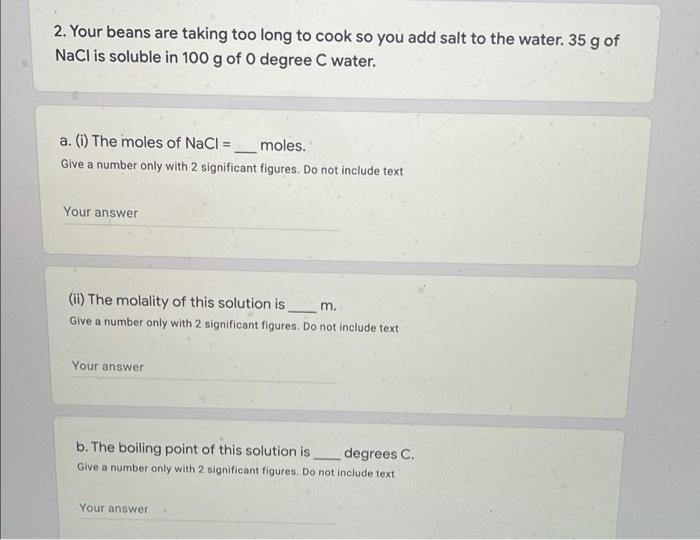
1. Adding a solute to a solvent __ the freezing point because the chemical forces between the makes it harder for the solvent to combine to form a solid. This energy is required to freeze the solution. means raises (blank 1) does not change (blank 1) lowers (blank 1) solute-solute (blank 2) solute-solvent (blank 2) solvent-solvent (blank 2) lower (blank 3) higher (blank 3) the same (blank 3) 2. Your beans are taking too long to cook so you add salt to the water. 35 g of NaCl is soluble in 100 g of 0 degree C water. a. (1) The moles of NaCl = ___ moles. Give a number only with 2 significant figures. Do not include text Your answer (ii) The molality of this solution is_m. Give a number only with 2 significant figures. Do not include text Your answer b. The boiling point of this solution is degrees C. Give a number only with 2 significant figures. Do not include text Your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


