Question: please help me answer the following questions Question 1 (1 point) Which one of the following gives a neutral aqueous solution? Mg(CIO4)2 OKF NaNO2 NH4Br
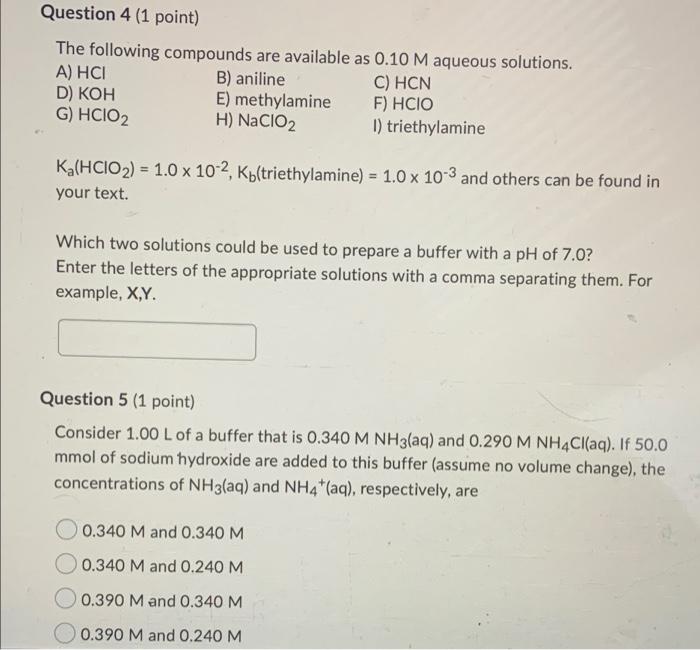
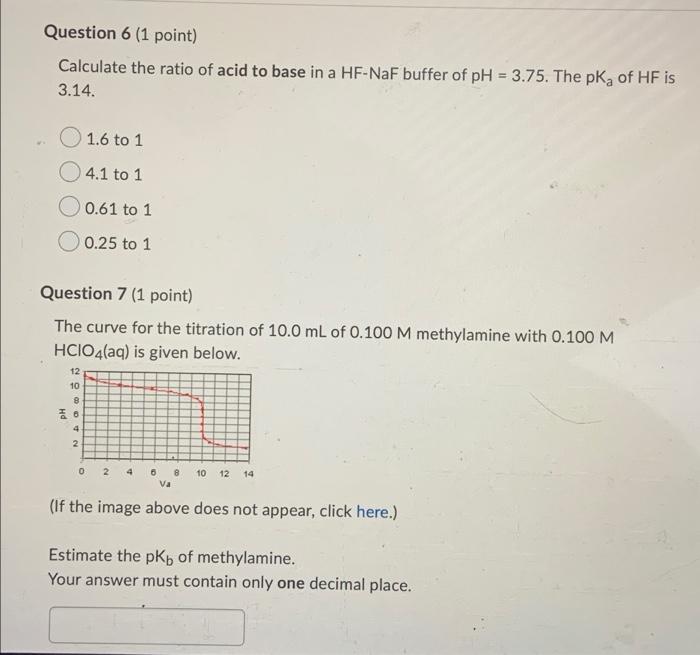

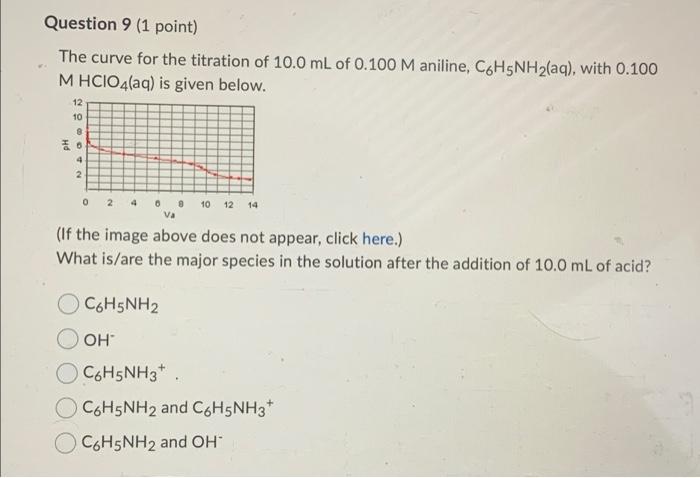

Question 1 (1 point) Which one of the following gives a neutral aqueous solution? Mg(CIO4)2 OKF NaNO2 NH4Br Question 2 (1 point) Calculate the pH of a 0.791 M aqueous solution of pyridinium chloride, CsH5NHCI. The Kb of pyridine is 1.8 x 10-? The answer must be given with the correct number of significant figures. Your Answer: Your Answer Question 4 (1 point) The following compounds are available as 0.10 M aqueous solutions. A) HCI B) aniline C) HCN D) KOH E) methylamine F) HCIO G) HCIO2 H) NaClO2 1) triethylamine Ka(HCIO2) = 1.0 x 10-2, Ktriethylamine) = 1.0 x 10-3 and others can be found in your text. Which two solutions could be used to prepare a buffer with a pH of 7.0? Enter the letters of the appropriate solutions with a comma separating them. For example, X,Y. Question 5 (1 point) Consider 1.00 L of a buffer that is 0.340 M NH3(aq) and 0.290 M NH4Cl(aq). If 50.0 mmol of sodium hydroxide are added to this buffer (assume no volume change), the concentrations of NH3(aq) and NH4+ (aq), respectively, are 0.340 M and 0.340 M 0.340 M and 0.240 M 0.390 M and 0.340 M 0.390 M and 0.240 M Question 6 (1 point) Calculate the ratio of acid to base in a HF-NaF buffer of pH = 3.75. The pk, of HF is 3.14. 1.6 to 1 4.1 to 1 O 0.61 to 1 0.25 to 1 Question 7 (1 point) The curve for the titration of 10.0 mL of 0.100 M methylamine with 0.100 M HCIO4(aq) is given below. 12 10 8 26 4 2 0 2 4 10 6 8 va 12 14 (If the image above does not appear, click here.) Estimate the pKb of methylamine. Your answer must contain only one decimal place. Question 8 (1 point) A weak base is titrated with hydrochloric acid solution. At the equivalence point, pH = pka. the maximum buffering capacity is achieved. pH = 7.0. pH > 7.0. OpH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


