Question: Please help me answer these two questions Consider the acid-base nature of ammonium nitrate, NH4NO3, when it is dissolved in water. (1) What are the
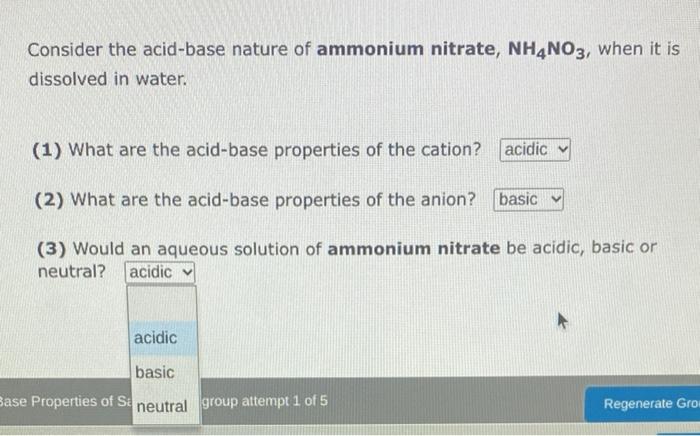
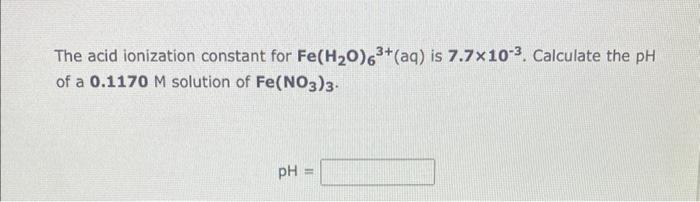
Consider the acid-base nature of ammonium nitrate, NH4NO3, when it is dissolved in water. (1) What are the acid-base properties of the cation? (2) What are the acid-base properties of the anion? (3) Would an aqueous solution of ammonium nitrate be acidic, basic or neutral? The acid ionization constant for Fe(H2O)63+(aq) is 7.7103. Calculate the pH of a 0.1170M solution of Fe(NO3)3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


