Question: Please help me :( CO(g) + 3H2(g) = CH4(g) + H2O(g) If the reaction above has a Keg of 72, the reaction is in favor

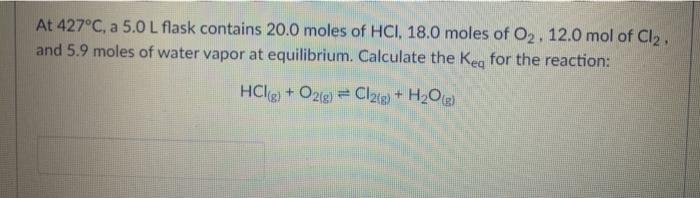
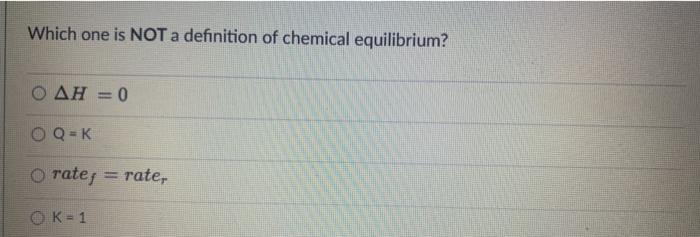
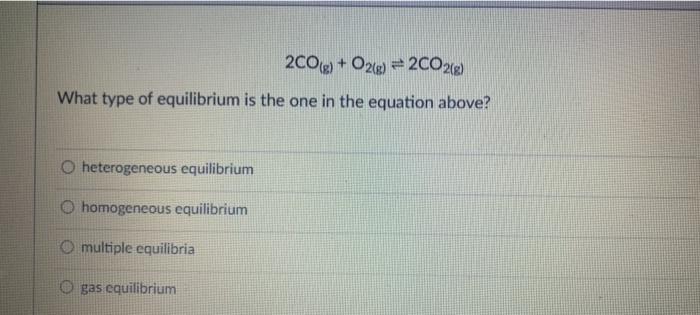

CO(g) + 3H2(g) = CH4(g) + H2O(g) If the reaction above has a Keg of 72, the reaction is in favor of: neither the reactants both reactants and products the products At 427C, a 5.0 L flask contains 20.0 moles of HCI, 18.0 moles of O2.12.0 mol of Cl2. and 5.9 moles of water vapor at equilibrium. Calculate the Keg for the reaction: HCls) + O2(g) + Cl2(g) + H2O) Which one is NOT a definition of chemical equilibrium? = 0 O Q=K O ratej rater OK= 1 2009 + O2(g) = 2002(0) What type of equilibrium is the one in the equation above? O heterogeneous equilibrium homogeneous equilibrium O multiple equilibria gas equilibrium A sample of ammonia gas and hydrogen chloride gas is placed in a sealed container at 25C and allowed to reach equilibrium according to the reaction: NH3(g) + HCLg) = NHACI) After equilibrium is established, the container was opened to allow NH3(g) and HCLg) to escape. How would this affect the equilibrium? The reaction would produce less NH3(g) and HCle The reaction would shift to the left to produce more NH3ig and HCl(a) The reaction would shift to the right to produce more NHACH The reaction would produce more NH3, HChaland NH4Cl until the original equilibrium is equilibrium re-established
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


