Question: please help me! Equation (1.2.5) for determining density (d) from mass (m) and volume (V) values is available in No Penalty Hint Eqn. hint (1.2.5).
 please help me!
please help me!
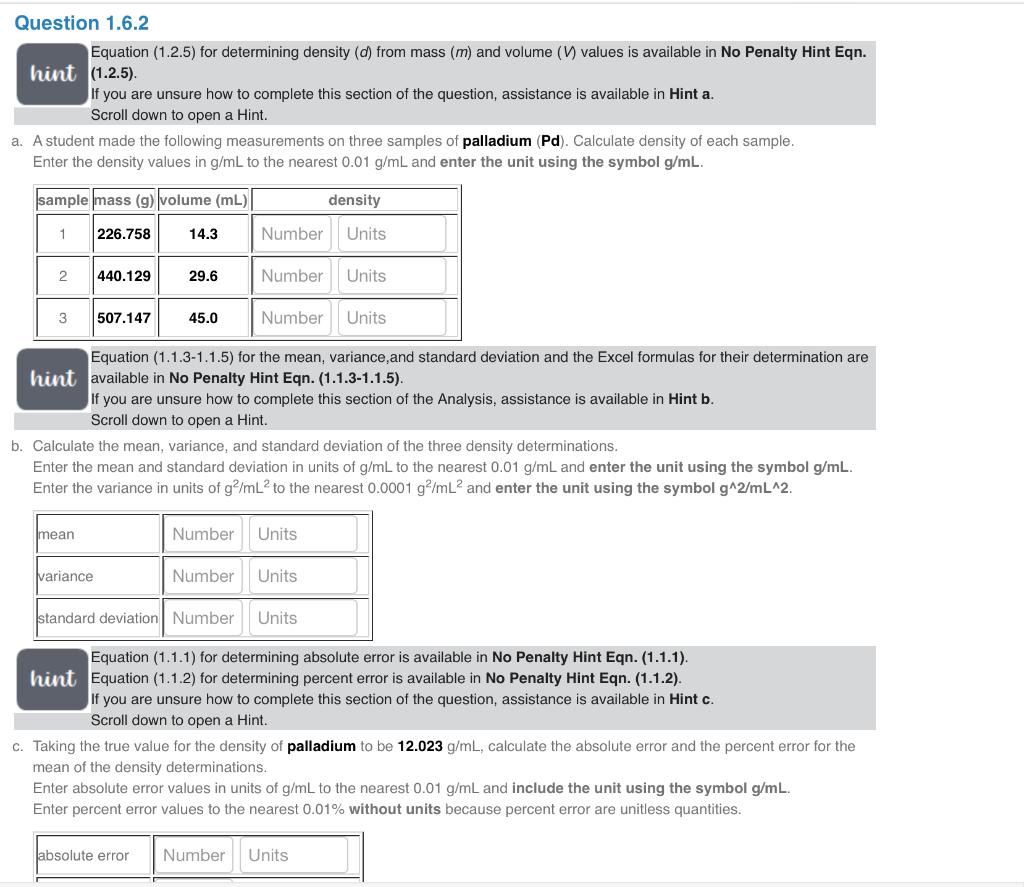
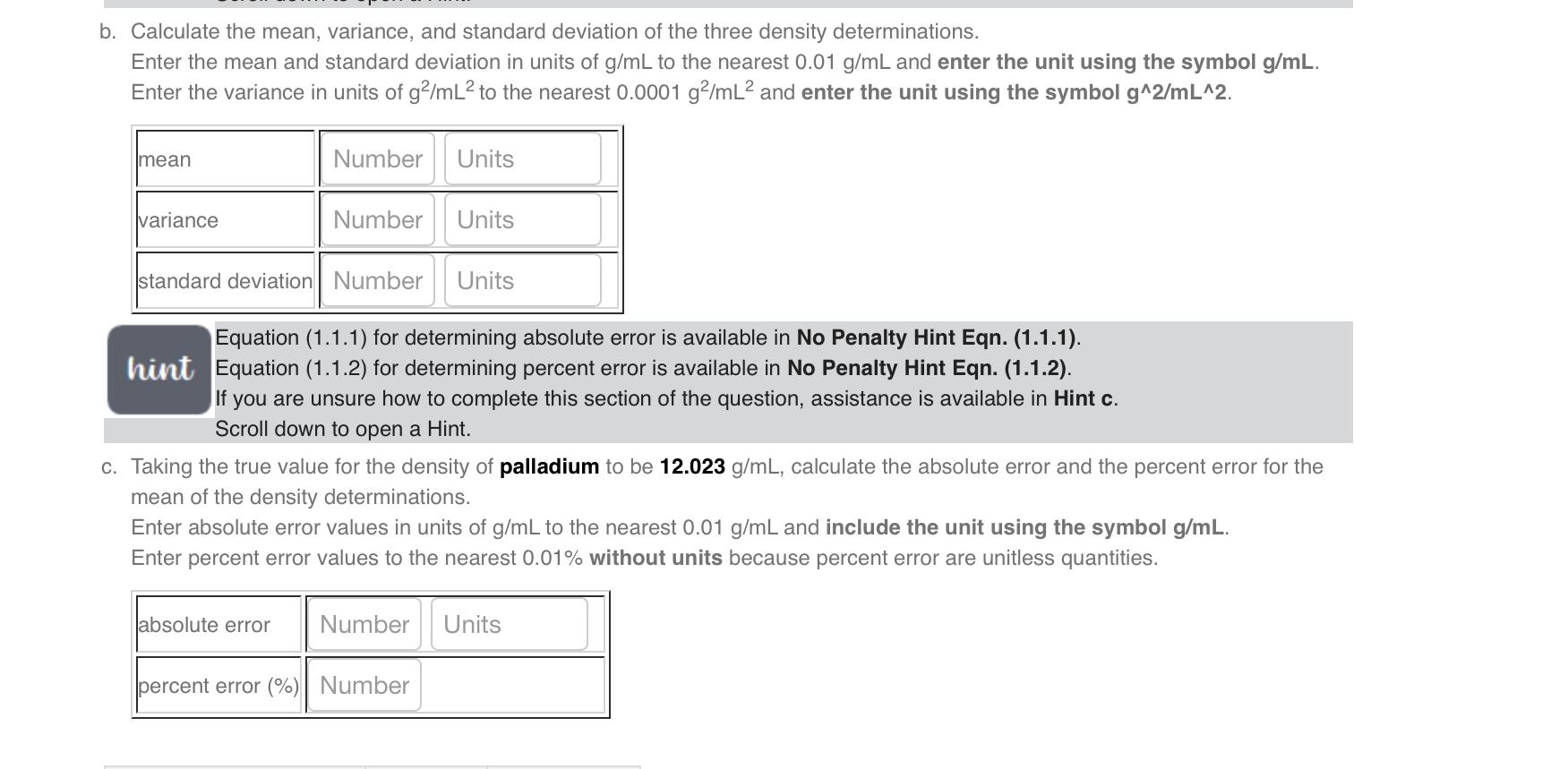
Equation (1.2.5) for determining density (d) from mass (m) and volume (V) values is available in No Penalty Hint Eqn. hint (1.2.5). If you are unsure how to complete this section of the question, assistance is available in Hint a. Scroll down to open a Hint. A student made the following measurements on three samples of palladium (Pd). Calculate density of each sample. Enter the density values in g/mL to the nearest 0.01g/mL and enter the unit using the symbol g/mL. Equation (1.1.3-1.1.5) for the mean, variance,and standard deviation and the Excel formulas for their determination are hint available in No Penalty Hint Eqn. (1.1.3-1.1.5). If you are unsure how to complete this section of the Analysis, assistance is available in Hint b. Scroll down to open a Hint. Calculate the mean, variance, and standard deviation of the three density determinations. Enter the mean and standard deviation in units of g/mL to the nearest 0.01g/mL and enter the unit using the symbol g/mL. Enter the variance in units of g2/mL2 to the nearest 0.0001g2/mL2 and enter the unit using the symbol g2/mL2. Equation (1.1.1) for determining absolute error is available in No Penalty Hint Eqn. (1.1.1). hint Equation (1.1.2) for determining percent error is available in No Penalty Hint Eqn. (1.1.2). If you are unsure how to complete this section of the question, assistance is available in Hint c. Scroll down to open a Hint. Taking the true value for the density of palladium to be 12.023g/mL, calculate the absolute error and the percent error for the mean of the density determinations. Enter absolute error values in units of g/mL to the nearest 0.01g/mL and include the unit using the symbol g/mL. Enter percent error values to the nearest 0.01% without units because percent error are unitless quantities. Calculate the mean, variance, and standard deviation of the three density determinations. Enter the mean and standard deviation in units of g/mL to the nearest 0.01g/mL and enter the unit using the symbol g/mL. Enter the variance in units of g2/mL2 to the nearest 0.0001g2/mL2 and enter the unit using the symbol g2/mL2. Equation (1.1.1) for determining absolute error is available in No Penalty Hint Eqn. (1.1.1). hint Equation (1.1.2) for determining percent error is available in No Penalty Hint Eqn. (1.1.2). If you are unsure how to complete this section of the question, assistance is available in Hint c. Scroll down to open a Hint. Taking the true value for the density of palladium to be 12.023g/mL, calculate the absolute error and the percent error for the mean of the density determinations. Enter absolute error values in units of g/mL to the nearest 0.01g/mL and include the unit using the symbol g/mL. Enter percent error values to the nearest 0.01% without units because percent error are unitless quantities
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


